DATE2021.08.06 #Press Releases
Elucidating the structure of the melatonin receptor signaling complex
Disclaimer: machine translated by DeepL which may contain errors.
~ Contribution to the understanding of the structural basis of sleep and circadian rhythms and to the development of sleep medications
Hiroyuki Okamoto (1st Year Doctoral Student, Department of Biological Sciences)
Asuka Inoue, Associate Professor, Tohoku University
Tomohiro Nishizawa (Associate Professor, Department of Biological Sciences (at the time of research) / Professor, Yokohama City University (current affiliation))
Osamu Nureki (Professor, Department of Biological Sciences)
Ryoji Hisano (Lecturer, Kansai Medical University)
Takuya Shimizu (Professor, Kansai Medical University)
Norimichi Nomura (Associate Professor, Kyoto University)
Sou Iwata (Professor, Kyoto University)
Key points of the presentation
- We have succeeded in clarifying the steric structure of the melatonin receptor (Note 2)MT1 signaling complex (Note 3 ), in which the sleeping drug ramelteon and Gi protein trimer (Note 1) are bound.
- We have identified the interaction with ramelteon, which is important for the activation of the receptor. Furthermore, by comparing the structure with other signaling complexes, we found spatial features on the intracellular side of the receptor that characterize the conjugation selectivity of G proteins.
- This study contributes to drug development targeting melatonin receptors and leads to an understanding of G-protein conjugate selectivity, the first step in GPCR (Note 4) signaling.
Summary of Presentation
Sleep is essential for the maintenance of our life and is regulated by various signaling molecules such as hormones. Melatonin (Note 5 ), the focus of this study, plays a particularly central role in the induction of sleep, and signals that inhibit neuronal activity by the melatonin receptor, a type of GPCR, and Gi protein trimer are important in this process. Melatonin receptors have attracted attention as a therapeutic target for sleep disorders, and the insomnia drug Ramelteon (brand name Rozerem) was approved in 2010. Therefore, structural determination of signaling complexes containing melatonin receptors will contribute not only to an atomic-level understanding of the mechanism of sleep, but also to the development of more effective drugs. Recently, crystal structures of melatonin receptors have been reported, but these structures show inactive conformations, and the mechanisms of structural changes upon activation of the melatonin receptor and its selective conjugation with the Gi protein trimer, a signaling factor, remain unknown.
In this study, Professor Osamu Nureki and his group at the Graduate School of Science, The University of Tokyo, clarified the three-dimensional structure of the signaling complex composed of the melatonin receptor MT1 and Gi protein trimer by single particle analysis using cryo-EM (Note 6). Furthermore, functional and bioinformatics analyses were conducted in collaboration with several domestic and overseas laboratories to clarify the activation mechanism of the receptor and the mechanism by which it selectively binds to the Gi protein trimer. The results of this research are expected to facilitate the development of therapeutic drugs for sleep disorders and advance research on the selective signaling between GPCRs and G proteins.
The results of this research were published in the scientific journal Nature Structural and Molecular Biology on August 5 (4 p.m. British Summer Time).
Publication details
Background of the research and problems in previous studies
Melatonin is a hormone secreted at night and involved in inducing sleep and controlling circadian rhythms (Note 7). Secreted melatonin binds to the melatonin receptor, a type of membrane receptor protein GPCR, and the melatonin receptor transmits inhibitory signals into the cell via the Gi protein trimer, ultimately resulting in physiological effects such as sleep induction. Because of the importance of these physiological effects, melatonin and melatonin receptors have attracted attention as therapeutic targets for sleep disorders and other disorders, and many agonists (Note 8) have been developed and used in clinical practice, but how these agents act on melatonin receptors to transmit signals has been less However, how these drugs act on melatonin receptors and transmit signals has not been well understood.
Recently, X-ray crystallography has reported the three-dimensional structure of the melatonin receptor with drugs for sleep disorders bound to it, and the recognition mechanism of the drugs has been elucidated. However, in a series of structural analyses, various mutations were introduced to stabilize the receptor, and mutants that did not show any physiological activity were used. Therefore, the structure of the melatonin receptor was in an inactive state, even though an agonist that activates the receptor was bound, and did not reflect the physiological state of the receptor. The mechanism by which the melatonin receptor is activated by the ligand remains unclear, and the detailed mechanism of action required for the development of therapeutic agents has not yet been elucidated.
The research group led by Graduate Student Hiroyuki Okamoto, Associate Professor Tomohiro Nishizawa, and Professor Osamu Nureki of the Graduate School of Science, The University of Tokyo, used cryo-EM single-particle analysis to identify the ligand-bound activation of the melatonin receptor MT1 and the Gi protein trimer. The structure of the signal transduction complex consisting of the ligand-bound and activated melatonin receptor MT1 and Gi protein trimer was elucidated using cryo-EM single particle analysis (Figure 1).
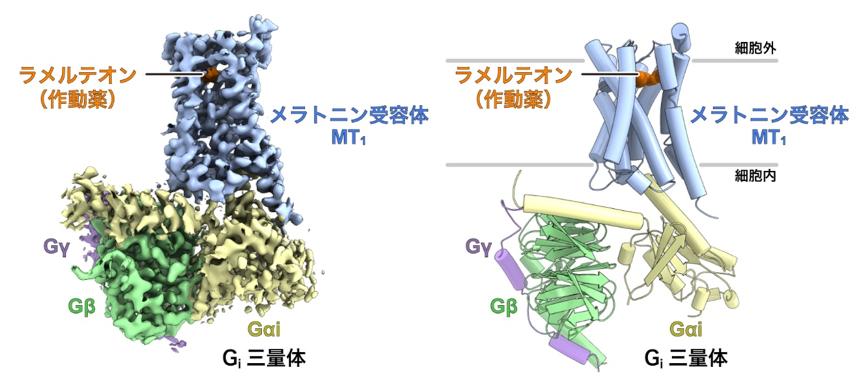
Figure 1: Overall structure of the melatonin receptor MT1-Gisignaling complex.
Left: Density map of the melatonin receptor MT1-Gisignaling complex.
Right: Structural model of the melatonin receptor MT1-Gisignaling complex constructed based on the density map.
This clarified the mechanism of melatonin receptor activation. Furthermore, by analyzing melatonin receptor mutants using the activation detection method for Gi protein trimer developed by Associate Professor Asuka Inoue of Tohoku University, we succeeded in identifying a new amino acid residue important for receptor activation that had not been identified in previous studies (Figure 2).
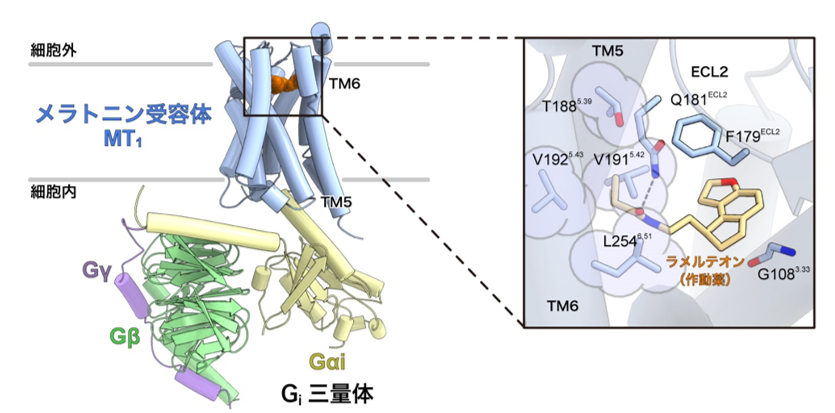
Figure 2: Recognition mechanism of the agonist ramelteon by the melatonin receptor MT1 in its activated state.
Left: Overall structure of the melatonin receptor MT1-Gisignaling complex.
Right: Enlarged view of the ligand binding site. The amino acid residues circled in light purple (T188, V191, V192, and L254) were found to be important for melatonin receptor activation for the first time. These amino acid residues were located in the fifth and sixth transmembrane helices (TM5 and TM6), near a group of amino acid residues called the "activation motif" that is conserved among many GPCRs.
There are several types of G proteins that are activated by GPCRs, and MT1 is known to selectively activate G proteins called Gi. GPCRs are generally known to undergo a conformational change at the sixth transmembrane helix (TM6) upon activation, but in the MT1 receptor We have found that TM6 moves in a more jump-like manner in the MT1 receptor than in other Gi signaling receptors (Note 4) (Fig. 3). Previous studies have shown that the conformational change of TM6 is smaller in Gi signaling receptors than in Gs signaling receptors (Note 4), and this difference is thought to determine the selectivity of the coactivating G protein. On the other hand, the conformational change of TM6 in the MT1 receptor revealed in this study was as large as that in the Gs-signaling receptor. Therefore, the movement of TM6 itself is not directly related to the selectivity of G protein signaling, suggesting that the degree of conformational change of TM6 is rather highly dependent on the distribution of hydrophobic amino acids in TM6.
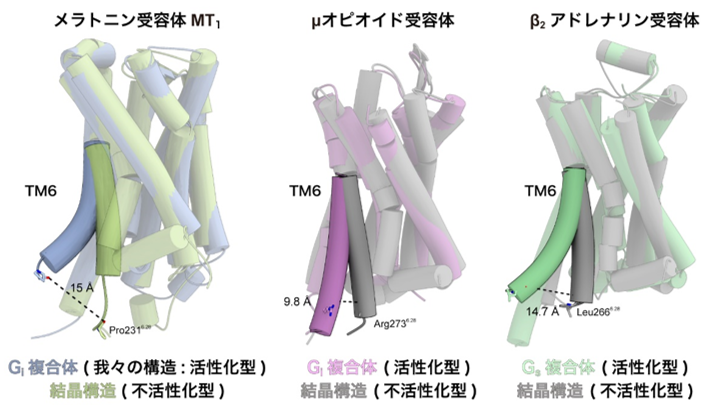
Figure 3: Comparison of the crystal structure of the inactivated form with that of the signaling complex
The crystal structures of TM6 for each of the melatonin receptor MT1 ( left), μ-opioid receptor (center), and β2-adrenergic receptor (right), taking the structure of the inactivated form, were compared with the structure of the signaling complex, which shows the structure of the activated state. The sixth transmembrane helix (TM6) of the melatonin receptor MT1 showed a greater conformational change than the other Gi signaling receptors and a jump to the same level as the Gs signaling receptors.
On the other hand, a comprehensive comparison of GPCR structures revealed that the space on the intracellular side is narrower in Gi signaling receptors than in Gs signaling receptors (Fig. 4). Furthermore, compared to the Gs-signaling receptor, the Gi-signaling receptor has a weaker interaction through the intracellular loop than the Gs-signaling receptor, indicating that the interaction occurs only at the C-terminus of Gi. Bioinformatics analysis using structural information by Associate Professor Raimondi of Scuola Normale Superiore di Pisa, Italy, revealed that G protein-receptor interactions are conserved among Gs signaling receptors, whereas G protein-receptor interactions are highly conserved among Gi signaling receptors. The results of bioinformatics analysis using structural information from the associate professor revealed that the G protein-receptor interaction is conserved among Gs-signaling receptors, while the interaction is more flexible among Gi-signaling receptors. In contrast to the conventional view that the selectivity between Gi-conjugated andGs-conjugated receptors is determined only by the difference in the degree of conformational change of TM6, it is clear that more factors such as the spatial characteristics of the intracellular side of the receptor and the interaction with G proteins through the intracellular loop contribute to the selectivity in a complex manner.
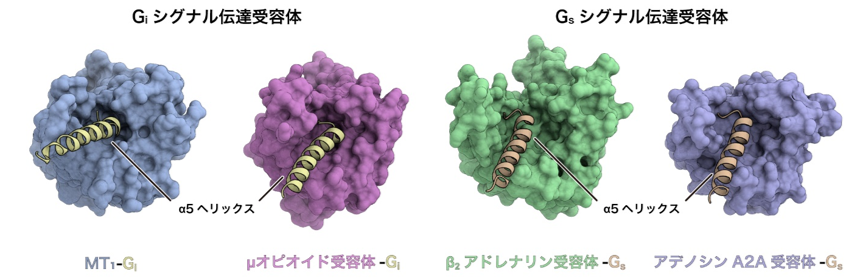
Figure 4: Comparison of the structures of Gi and Gs signaling receptors.
The structures of Gi signaling receptors (left: melatonin receptor MT1, right: μ opioid receptor) and Gs signaling receptors (left: β2 adrenergic receptor, right: adenosine A2A receptor) are compared from the intracellular side. The Gi signaling receptor has a narrow intracellular space, while the Gs signaling receptor has a relatively large intracellular space.
Social Significance and Future Plans
By combining the three-dimensional structure of the activated melatonin receptor in this study with the three-dimensional structure of the inactive melatonin receptor obtained by X-ray crystallography in our previous study, the search for drugs for melatonin receptors will be accelerated by computer simulation, and the development of drugs for insomnia and circadian rhythm disturbances such as jet lag will be accelerated. This research is expected to accelerate the drug discovery of melatonin receptors by computer simulation and lead to the development of therapeutic drugs for insomnia and circadian rhythm disorders such as jet lag.
This research was conducted as part of the Grant-in-Aid for Specially Promoted Research "Elucidation of Molecular Mechanisms of Membrane Proteins Regulated by Physical Stimuli" (PI: Osamu Nureki) and the Young Scientist (A) "Study of Structural Basis for Ligand Diversity of G Protein-Coupled Receptors" (PI: Tomohiro Nishizawa) funded by the Japan Society for the Promotion of Science (JSPS). The research was also conducted as part of the National This research was also conducted as part of the "Platform for Supporting Life Science Research including Drug Discovery Initiative" of the Japan Agency for Medical Research and Development (AMED), which aims to link the results of outstanding life science research to the practical application of pharmaceuticals and other products by opening up large facilities such as cryo-electron microscopes to the outside world. The project was supported by the "Platform for Advanced Technology Support (BINDS)" and the "Innovative Research and Development Support Project" Solo Type (PRIME).
Journal
-
Journal name Nature Structural and Molecular Biology Title of paper Cryo-EM structure of the human MT1-Gi signaling complex Author(s) Hiroyuki H. Okamoto, Hirotake Miyauchi, Asuka Inoue*, Francesco Raimondi, Hirokazu Tsujimoto, Tsukasa Kusakizako, Wataru Shihoya, Keitaro Yamashita, Ryoji Suno, Norimichi Nomura, Takuya Kobayashi, So Iwata, Tomohiro Nishizawa*, Osamu Nureki* (*: joint authors) DOI Number 10.1038/s41594-021-00634-1
URL of the paper
Terminology
Note 1 Gi protein tr imer , G protein trimer
G protein is a GTP-binding protein involved in intracellular signaling and is composed of a trimer of Gα, Gβ, and Gγ subunits, which is activated by an activated GPCR (Note 5). The activated G protein trimer undergoes a GDP-GTP exchange reaction and dissociates into two subunits, Gα and Gβ-Gγ. The Gα subunit is divided into four major subunits, Gs , Gi ,Gq/11, andG12/13, and the Gi protein trimer in particular inhibits the activity of adenylate cyclase downstream, resulting in an inhibitory signaling response. inhibits the activity of adenylate cyclase downstream, thereby transmitting an inhibitory signal. ↑up
Note 2 Melatonin receptor
The melatonin receptor is classified as a Class A GPCR, which binds small molecule ligands and is a major traditional drug target. Binding of melatonin results in an activated state, selectively activating an inhibitory G protein trimer (Gi protein trimer) that inactivates adenylate cyclase. There are two subtypes of melatonin receptors, MT1 and MT2, and MT1, expressed mainly in the suprachiasmatic nucleus of the brain, is known to play a particularly important role in inducing sleep. ↑up
Note 3 Signaling complexes
Activated GPCRs form a signaling complex by binding to G protein trimer and are stabilized in a conformation that facilitates the GDP-GTP exchange reaction at the G protein trimer. With the development of single-particle analysis methods using cryo-EM, many steric structures of GPCR signaling complexes have been reported. ↑up
Note 4 GPCR (G protein-coupled receptor), Gi signaling receptor, Gs signaling receptor
GPCRs are membrane proteins composed of seven α-helices and form the largest family of membrane receptor proteins, with the N-terminus located extracellularly and the C-terminus intracellularly, and are activated by the binding of specific ligands in the extracellular region to activate the intracellular G protein trimer, thereby G-protein trimer in the cell. Because of their important role in regulating diverse physiological functions in the body, more than 30% of approved drugs target GPCRs. Among these, those that conjugate with Gi protein trimer are called Gi signaling receptors and those that conjugate with Gs protein trimer are called Gs signaling receptors. ↑up
Note 5 Melatonin
Melatonin is a hormone synthesized in the pineal gland of the brain via serotonin from tryptophan as a starting substance. Melatonin was discovered by Lerner et al. in 1958, and previous studies have shown that it is synthesized in large amounts at night and is involved in sleep induction and the regulation of circadian rhythms. Because of their involvement in these important physiological phenomena, melatonin and melatonin receptors (Note 2) have attracted attention as therapeutic targets for sleep disorders and other disorders, and compounds similar to melatonin have been developed for the treatment of sleep disorders. ↑up
Note 6 Single particle analysis method using cryo-electron microscopy
This is a method to determine the three-dimensional structure of a biopolymer sample such as a protein by photographing the sample using a cryo-electron microscope and reconstructing the three-dimensional structure by image processing a large number of photographed images. The sample is observed and photographed by irradiating electron beams to the biopolymer sample such as protein under liquid nitrogen (-196°C) cooling. Remarkable technological innovations in detectors and other devices have made it a popular method for determining the steric structure of proteins and other biological macromolecules at high resolution, and in 2017, the Nobel Prize in Chemistry was awarded to three overseas researchers who contributed to its development. ↑up
Note 7 Circadian rhythm
A physiological phenomenon that fluctuates in cycles of approximately 25 hours and is conserved in most living organisms, including animals, plants, fungi, and algae. Circadian rhythms are phenomena that occur even in the absence of periodic fluctuations in the environment, such as light, and are involved in many vital phenomena, including sleep and wakefulness, and play an important role in living organisms. ↑up
Note 8 Agonist
Agonists are drugs that act as ligands for receptor proteins and enhance the activity of the receptors through binding. In particular, the physiological hormone melatonin also acts as an agonist at the melatonin receptor, and many drugs for sleep disorders that target the melatonin receptor are agonists. ↑


