DATE2021.07.27 #Press Releases
Elucidating the steric structure of the last remaining beta-adrenergic receptor
Disclaimer: machine translated by DeepL which may contain errors.
〜Contributing to the Discovery of Beta-Adrenergic Receptor-Targeted Drugs with Fewer Side Effects
Chisae Nakiri (2nd year, Master's course, Department of Biological Sciences)
Kazuhiro Kobayashi (Department of Biological Sciences, 2nd year of Doctoral program)
Atsuhiro Tomita (Department of Biological Sciences, 3rd year of Doctoral program)
Masahiko Kato (1st year, Master's course, Department of Biological Sciences)
Tomohiro Nishizawa (Associate Professor, Department of Biological Sciences)
Asuka Inoue (Associate Professor, Tohoku University)
Wataru Shihoya (Assistant Professor, Department of Biological Sciences)
Osamu Nureki (Professor, Department of Biological Sciences)
Key points of the presentation
- Among the three β-adrenergic receptors (Note 1 ), which are important drug target molecules, the three-dimensional structure of the complex between the β3 receptor and Mirabegron, a drug for overactive bladder (OAB), was clarified.
- The drug-binding pocket of the β3 receptor is narrower than that of other β-adrenergic receptors, and Mirabegron can selectively bind to the β3 receptor by fitting into the narrow pocket.
- The visualization of the steric structures of all β-adrenergic receptors in this study is expected to accelerate the development of β-adrenergic receptor-targeted drugs with fewer side effects.
Summary of Presentation
Adrenaline stimulates sympathetic nerves by activating β-adrenergic receptors, which are G-protein coupled receptors (GPCRs) (Note 2 ), and increases heart rate and blood pressure. There are three types of β-adrenergic receptors, β1-3, and drugs targeting β1 and β2 are typical drugs for heart disease and asthma. On the other hand, β3 receptors are abundantly expressed in adipocytes and are responsible for heat production and lipolysis. Polymorphisms in the β3 receptor gene are known as thrifty genes (Note 3 ) because they lower basal metabolism and make people more likely to gain weight. β3 receptors are also involved in smooth muscle relaxation of the bladder, and mirabegron (product name Benidus), a selective stimulator of β3 receptors, is a treatment for overactive bladder (Note 4). Because β1-3 receptors have different physiological effects, the selectivity of the drug for each receptor is important for reducing side effects. So far, research on β1 and β2 receptors has progressed, and many atomic-level structures have been elucidated to understand the mechanism of action of the drugs. However, only the steric structure of the β3 receptor has not been elucidated, and our understanding of the selectivity of β-receptor stimulating drugs has been limited.
In this study, a group led by Professor Nureki at The University of Tokyo Graduate School of Science, in collaboration with Associate Professor Asuka Inoue at Tohoku University Graduate School of Pharmaceutical Sciences, determined the steric structure of the β3 receptor bound by the overactive bladder drug Mirabegron by single particle analysis using a cryo-EM (Note 5). In the structure, Mirabegron is elongated and sits vertically in the drug-binding pocket of the β3 receptor, visualizing the details of the interaction between Mirabegron and the β3 receptor. The entrance to the drug-binding pocket of the β3 receptor is narrower than that of the β1 and β2 receptors, and the entire pocket is linear in shape. These differences in pocket shape were found to be important for Mirabegron's β3 receptor selectivity. Now that the steric structures of all β-adrenergic receptors have been clarified, it is expected that β-adrenergic receptor-targeted drugs with higher selectivity for each receptor and fewer side effects will be created.
The research results were published in the American scientific journal Molecular Cell on July 27, 2021 (Japan time).
Publication details
Background of the research
Adrenaline is a hormone that controls the human instinct of "fight or flight" and stimulates the sympathetic nervous system to increase heart rate and blood pressure. Adrenaline exerts its excitatory effects by activating beta-adrenergic receptors, which are GPCRs. There are three types of β-adrenergic receptors, β1-3, which have very different physiological roles (Figure 1).
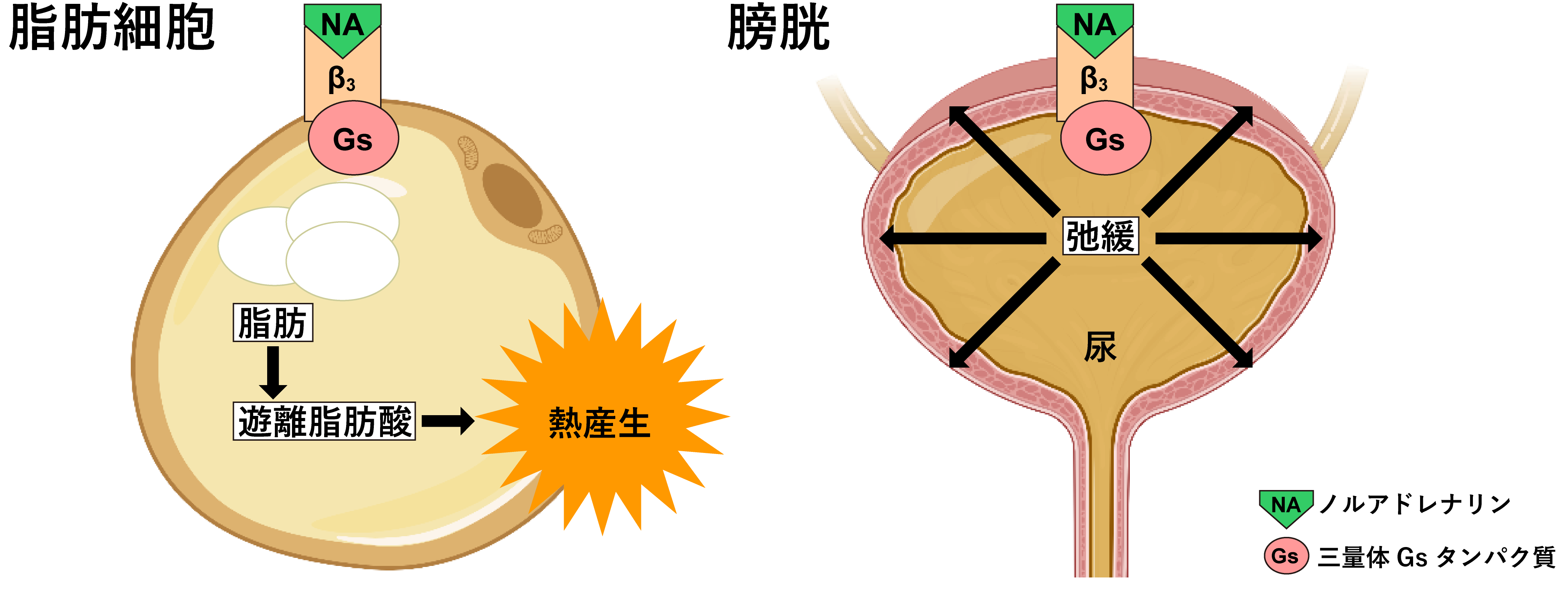
Figure 1: Typical functions of β3 receptors
β1 receptors are expressed in the myocardium and control heart rate. β1 blockers, which inhibit β1 receptors and reduce heart rate and blood pressure, are therapeutic agents for heart failure. On the other hand, β2 receptors are involved in smooth muscle relaxation of blood vessels and bronchial tubes, and β2 stimulants are typical treatments for bronchial asthma. The β1 and β2 receptors were the first GPCRs to be discovered and have boosted the research field as the first runner in GPCR research. The atomic-level three-dimensional structure of the receptors was also elucidated for the first time for hormone receptors, and the mode of drug binding and the principle of receptor operation are most well understood. His work on the β-adrenergic receptor was the subject of the 2012 Nobel Prize in Chemistry (Note 6).
The β3 receptor is the last β-adrenergic receptor to be identified and differs in function from the β1 and β2 receptors. β3 receptors are activated by noradrenaline and exert their physiological effects by activating downstream signaling. β3 receptors are abundantly expressed in adipocytes and are responsible for heat production and lipolysis (Figure 1). Polymorphisms (single amino acid residue substitutions) exist in the β3 receptor gene. Polymorphic β3 receptors, known as thrifty genes, have reduced function, and humans with these genes have an average basal metabolic rate of 150 kcal less. One in three Japanese has this thrifty mutation, which makes it difficult to lose weight and is a risk factor for diabetes and obesity in today's saturated diet. For this reason, β3 receptor stimulants have been studied as drugs for obesity and diabetes. β3 receptors are also involved in smooth muscle relaxation in the digestive organs, and are particularly abundant in the bladder (Figure 1). Activation of β3 receptors can relax the bladder and inhibit urine discharge, and Mirabegron (product name Benidus), a selective β3 receptor stimulator developed by Astellas Pharma, is a treatment for overactive bladder.
Thus, because β1-3 have different physiological effects, the selectivity of the drug for each receptor is important in reducing side effects. In fact, mirabegron is contraindicated in patients with severe cardiac disease because it increases heart rate through direct or indirect activation of β1 receptors. To understand β1-3 selectivity, it is necessary to visualize how selective drugs bind to the receptors. In the 13 years since the structures of the β1 and β2 receptors have been elucidated, only the steric structure of the β3 receptor has remained unresolved. Therefore, our understanding of the selectivity of β-receptor stimulants has been limited.
Research and Results
The research group first attempted to analyze the structure of the human-derived β3 receptor, but it was difficult to purify and analyze. After various investigations, they found that canine-derived β3 receptors are stable and optimal for structural analysis. Using cryo-EM single-particle analysis, we determined the three-dimensional structure of the signaling complex composed of mirabegron, canine β3 receptor, and trimeric Gs protein (Fig. 2). This has elucidated the binding mode of mirabegron and the signaling mechanism by the activated receptor.
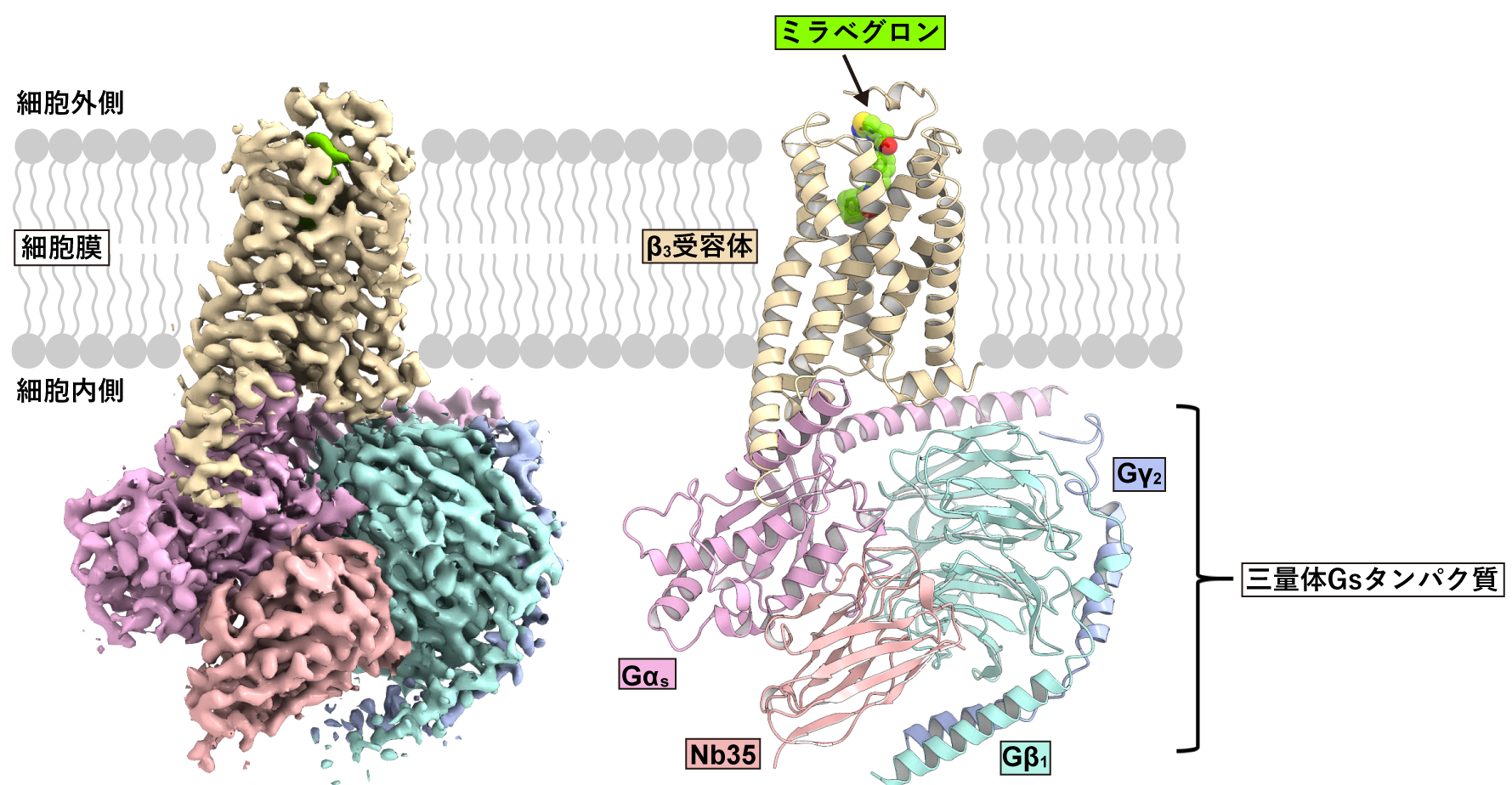
Figure 2: Overall structure of the signaling complex of the β3-adrenergic receptor.
On the left is the density map obtained by single-particle analysis. On the right is the three-dimensional structure of the signaling complex modeled based on the density map.
Mirabegron is a drug composed of parts that mimic noradrenaline and parts that do not (Figure 3 left). Mirabegron was elongated and fit vertically within the drug-binding pocket of the β3 receptor (Figure 3 right). The noradrenaline-like portion of the mirabegron interacted with the noradrenaline binding site, while the tail interacted with the extracellular pocket entrance of the binding pocket. In total, 17 amino acid residues interacted with mirabegron, and all but one were conserved between human and canine β3 receptors. These facts predicted that mirabegron would bind to human β3 receptors as well.
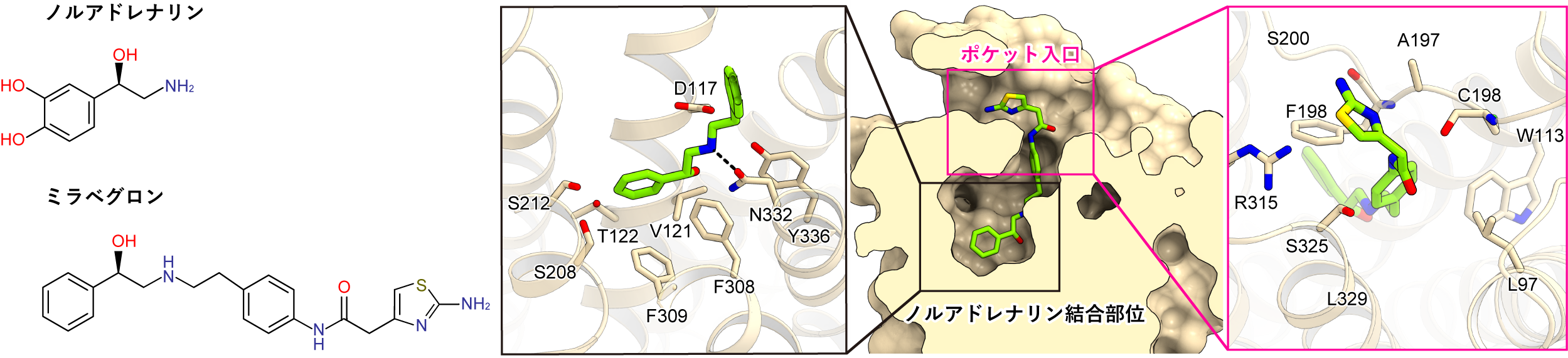
Figure 3: Binding Mode of Mirabegron
(left) Chemical structure of mirabeguron, (right) Cross section of the mirabeguron binding pocket and interaction.
Next, to investigate why mirabegron can selectively activate β3 receptors, we compared the structure of β3 receptors with that of β1 and β2 receptors (Figure 4). The amino acid residues that form the noradrenaline binding site were conserved among β-receptors, and the mode of drug binding was similar. In contrast, the amino acid residues shaping the pocket entry points were less conserved and the entry points differed greatly. The drug-binding pockets of β1 and β2 receptors have an open structure, and the selective drug tails interact differently with Mirabegron. In contrast, the pocket entrances of the extracellular-facing β3 receptors were narrower than those of the β1 and β2 receptors. Mirabegron can bind with high affinity to the narrow drug-binding pocket of the β3 receptor and cannot bind well to the β1 and β2 receptors, which have an open entrance. Conversely, β1- and β2-selective stimulants cause steric hindrance to the narrow drug-binding pocket of the β3 receptor. Thus, the narrow entrance of the drug-binding pocket at the β3 receptor is thought to be responsible for the β3 receptor selectivity of mirabegron.
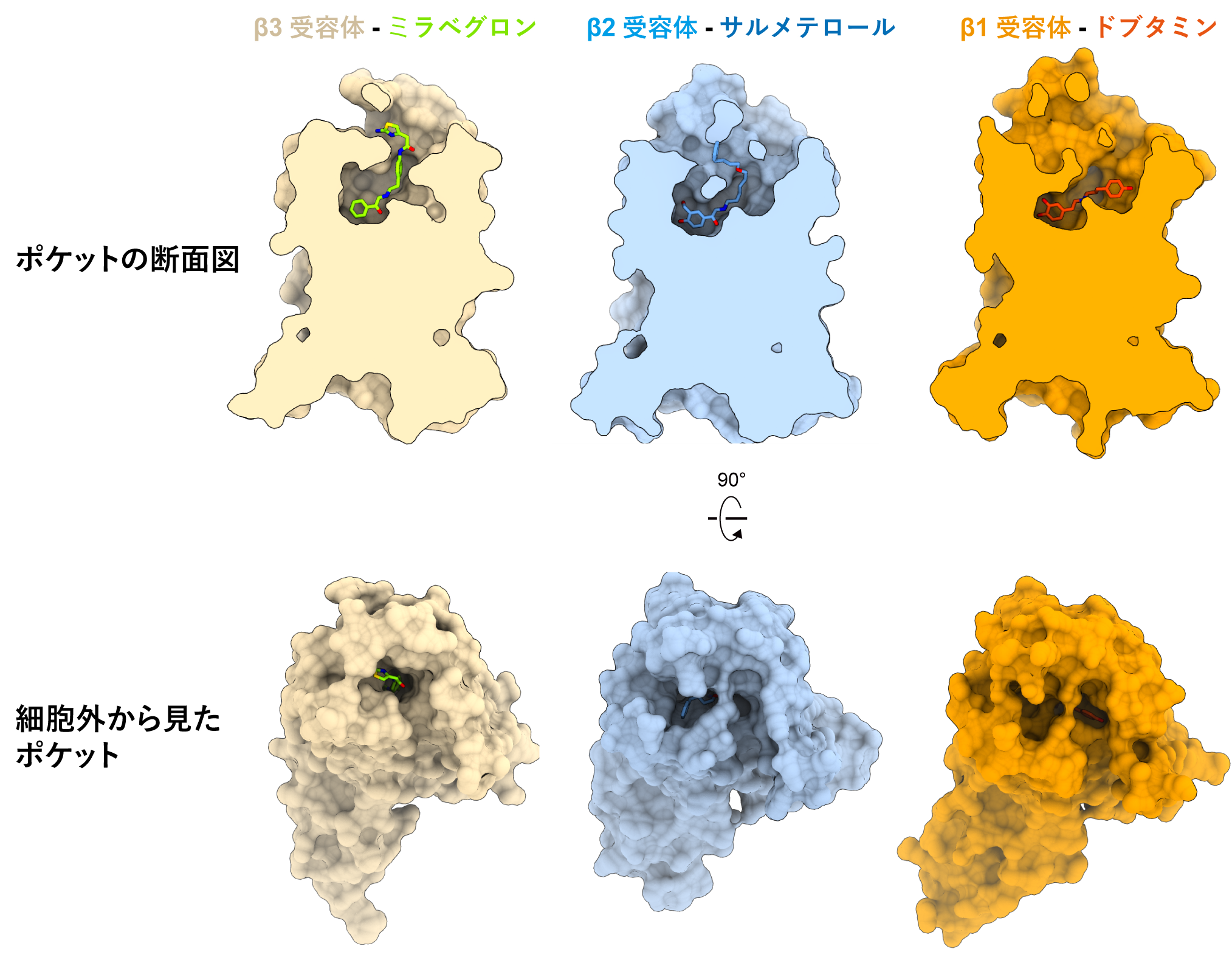
Figure 4: Structural comparison of β-adrenergic receptors
The structures of the β1, β2, and β3 receptors and their receptor-selective drug-bound structures are compared.
This research was supported by Grant-in-Aid for Specially Promoted Research "Elucidation of Molecular Mechanisms of Membrane Proteins Regulated by Physical Stimuli" (Project No. 16H06294, PI: Osamu Nureki) from the Japan Society for the Promotion of Science, and by the Uehara Memorial Life Science Foundation Research Grant "Single Particle Analysis of β3 Adrenoceptor" ( The research was conducted as part of the "Functional Elucidation of Lipid-Associated Orphan Receptors Using Ligand-Free Innovative GPCR Tools" (project number: 19gm5910013, PI: Asuka Inoue), a solo research project supported by the Japan Agency for Medical Research and Development (AMED). The research was conducted as a part of the project. This research was also conducted as part of the AMED "Platform for Supporting Life Science Research including Drug Discovery" and "Incubate Type of Support Program for Innovative R&D on Advanced Technology," which aims to link the results of outstanding life science research to the practical application of pharmaceuticals and other products by opening large facilities such as synchrotron radiation facilities to external users. The project was supported by the Platform for Advanced Technology Support for Drug Discovery, etc. (BINDS).
Future Prospects
This study shows that β3 receptor selectivity is achieved by successfully fitting the drug into the narrow drug-binding pocket of the β3 receptor. Based on the structural information, drugs with enhanced β3 receptor selectivity are expected to be developed, leading to the development of drugs for the treatment of overactive bladder with fewer side effects, and furthermore, drugs for the treatment of obesity and diabetes. In addition, now that the entire structure of the adrenergic receptor has been clarified, drug discovery targeting adrenergic receptors as a whole is expected to be revitalized.
Journal of Publication
-
Journal name Molecular CellTitle of paper Cryo-EM structure of the β3 adrenergic receptor reveals the molecular basis of subtype selectivity .Author(s) Chisae Nagiri, Kazuhiro Kobayashi, Atsuhiro Tomita, Masahiko Kato, Kan Kobayashi, Keitaro Yamashita, Tomohiro Nishizawa, Asuka Inoue*, Wataru Shihoya*, Osamu Nureki*.DOI Number
Terminology
1 Adrenergic receptor
They are found primarily in cardiac and smooth muscle, but also in brain and adipocytes. Adrenergic receptors are currently classified into three types, α1, α2, and β, and three further subtypes each. ↑
Note 2 G-protein coupled receptor (GPCR)
GPCRs are representative receptor proteins that exist in the plasma membrane. When they bind to signal molecules in the extracellular portion, they change the structure of the intracellular portion and activate trimeric G proteins. There are approximately 800 types of GPCRs in humans, and they are involved in various biological phenomena, making them important drug targets. ↑up
Note 3: Thrift gene
A genetic mutation that has emerged in the human race's struggle against hunger to store energy efficiently in fat. People with the thrifty gene used less energy in their daily lives than those who did not have it, which allowed them to survive even in times of famine and gave them an advantage for survival. However, in today's society, where saturated food and lack of exercise are chronic factors, it is easy to gain fat and is a risk factor for obesity and diabetes. β3 receptor mutations are the most representative thrifty genes among the 40 types that exist, and are also used as criteria in determining genetic testing. The proportion of thrifty mutations varies widely among races and correlates with the risk of obesity and diabetes. Needless to say, lifestyle is a major risk for obesity and diabetes, not just genetic background. ↑up
Note 4 Overactive bladder
Overactive bladder is a disease in which the bladder contracts regardless of one's intention, even when there is not enough urine in the bladder. It is one of urinary problems, and symptoms such as frequent urination and urinary urgency are manifested. Although the detailed mechanism of the onset of the disease is not known, it is believed to be caused by a failure in the communication between the brain, urethra, and bladder muscles through nerves. ↑up
Note 5 Cryo-electron microscopy
A device used to observe samples by irradiating electron beams to biomolecules such as proteins under liquid nitrogen (-196°C) cooling. As a method for determining the three-dimensional structure of proteins at high resolution, it has achieved remarkable technological innovation in detectors, etc. In 2017, the Nobel Prize in Chemistry was awarded to three overseas researchers who contributed to its development. ↑up
Note 6: The 2012 Nobel Prize in Chemistry
The 2012 Nobel Prize in Chemistry was awarded to Robert Lefkowitz and Brian Kobylka for studies of G-protein-coupled receptors (GPCRs). Lefkowitz isolated the beta-adrenergic receptor, elucidated its mechanism of action, and identified the gene that encodes it. This gene is very similar to the gene sequence of receptors found throughout the body, and this clarification led to a growing understanding that receptors that should be located elsewhere in the body are GPCRs working in a similar structure and mechanism. Brian Kobylka worked on the adrenergic receptor in Lefkowitz's group, and then went on to analyze the three-dimensional structural information. In 2011, the structure of the β2 receptor in complex with a G protein similar to that in this study was reported, providing details of the mechanism of receptor activation by adrenaline. ↑up


