DATE2021.06.24 #Press Releases
Hydrogen dissolution into iron was inhibited by sulfur in the early stages of earth formation
Disclaimer: machine translated by DeepL which may contain errors.
Riko Iizuka (Visiting Researcher, Geochemical Research Center / Project Assistant Professor at the time of the research)
Hiromasa Goto (Technical Specialist, Institute for Solid State Physics)
Chikara Ichihigashi (Department of Earth and Planetary Science, 2nd year of Master course at the time of research)
Koh Fukuyama (PD, Ehime University / at the time of research: 3rd year of Doctoral Program, Department of Earth and Planetary Science)
Yuichiro Mori (Department of Earth and Planetary Science, 2nd year of Master course)
Takanori HATTORI (Senior Staff, Japan Atomic Energy Agency)
Asami Sano (Senior Staff, Japan Atomic Energy Agency)
Kenichi Funakoshi (Senior Staff, Institute of Engineering Innovation)
Hiroyuki Kagi (Professor, Geochemical Research Center)
Key points of the presentation
- Neutron diffraction (Note 1) experiments under high temperature and high pressure simulating the early stage of Earth formation were conducted to determine the amount of hydrogen incorporated into iron. The results show that the coexistence of sulfur suppresses the hydrogenation (Note 2) of iron.
- Among the candidate light elements in the iron-dominated Earth's core, the mutual influence of hydrogen and sulfur on their dissolution into the Earth's core was clarified. The results indicate that hydrogen and sulfur may have reacted preferentially with solid iron during the early stages of Earth formation, after which other light elements may have been more readily dissolved into iron.
- The results of this study are expected to advance our understanding of the behavior of light elements during the formation of the primitive Earth, especially the process of microplanetary accumulation to core-mantle differentiation (Note 3).
Summary of presentation
Several light elements (H, C, O, Si, S, etc.) are thought to be dissolved in the iron-based core of the Earth. However, what kind of light elements exist and to what extent, and how they entered the core have not been clarified, and many experimental and theoretical studies have been conducted. Hydrogen, one of the most promising candidates for light elements, may have been incorporated into iron from water, which existed in large amounts in the primitive Earth in the early stages of Earth formation. However, it has not been clarified how its quantity and process are affected by other light elements.
A research group at the Geochemical Research Center, led by Senior Specialist Riko Iizuka of the Graduate School of Science, The University of Tokyo, in collaboration with Technical Specialist Hiromasa Goto of the Institute for Solid State Physics, The University of Tokyo, the J-PARC Center of the Japan Atomic Energy Agency, and the Institute of Engineering Innovation, conducted a high temperature and high pressure experiment simulating the early stage of Earth formation to investigate the process of light elements incorporated into iron. The process of incorporation of light elements into iron was observed in situ by neutron diffraction. As a result, it was revealed that the hydrogenation of iron, which occurs in the reaction between water dehydrated from hydrous minerals and iron under high temperature and high pressure, is suppressed by coexisting iron sulfide. This suggests that hydrogen and sulfur were preferentially dissolved into iron in the solid state, and that other light elements were likely dissolved into the molten iron afterward.
Publication details
Background of Research
The present-day Earth's core has a smaller density than that assumed for pure iron Fe, and is thought to contain dissolved light elements such as hydrogen H, carbon C, oxygen O, silicon Si, and sulfur S. Our previous studies had shown that in Fe-hydrous silicate samples simulating Earth's primordial material, Fe hydrides undergo a redox reaction between solid Fe and water produced by the decomposition of hydrous minerals (see 2017 press release https://www.s.u - tokyo.ac.jp/en/press/2017/5210/#a3). However, the contribution of other light elements to this hydrogenation had not been clarified.
Research Details
In this study, we focused on sulfur, which is abundant in primitive iron meteorites and in the cores of Mars and other planets and, like hydrogen, is a factor that significantly reduces the density and melting point of iron. Neutron diffraction experiments under high temperature and high pressure were conducted on samples that simulated the composition of the primitive Earth to clarify the effect of sulfur on the hydrogenation of iron.
The experiments were conducted using a large 6-axis press, "Pressing Princess," installed at the high-pressure beamline PLANET (Note 4) in the Materials and Life Science Experimental Facility MLF at J-PARC. To simulate the conditions of early Earth formation, the sample capsule was filled with a powder mixture (quartz SiO2 and brucite Mg(OH) 2 ) of iron, sulfur, and hydrous silicate, which are the primordial materials of the Earth (in the actual experiment, deuterium (D) was substituted for hydrogen to reduce background in the diffraction patterns). Mg(OD) 2 substituted with deuterium (D) was used in the actual experiments (Note 1) ). For comparison, experiments were also performed with a sulfur-free sample, and diffraction measurements were performed in the temperature and pressure range from 6-12 GPa and up to 1200 K. The sample was pressurized and then subjected to a stepwise pressure drop. Pressurizing the sample and then heating it in stages dehydrates the Mg(OH) 2 to form water, which reacts with the high-temperature, high-pressure phase of iron to form iron hydride. If the starting sample contained sulfur, iron sulfide (FeS) was formed together with the high-temperature and high-pressure phase of iron, and no hydrogen was incorporated into this FeS. Rietveld analysis (Note 5) of the obtained diffraction pattern (Fig. 1) to calculate the amount of hydrogen incorporated into iron showed that the sulfur-containing sample had less hydrogen than the sulfur-free sample at all temperatures and pressures (Fig. 2). These results clearly indicate that when sulfur is included, the FeS formed by the reaction with iron inhibits the hydrogenation of iron.
In addition, the present results are different from the results of previous studies on samples without hydrous minerals (direct reaction of FeS alloy with hydrogen produces hydrogenated FeS), suggesting that the water produced during the experiment affects the mechanism of the hydrogenation reaction and the phase equilibrium relationship between iron hydride and iron sulfide. The water produced during the experiment may affect the mechanism of the hydrogenation reaction and the phase equilibrium relationship between iron hydride and iron sulfide.
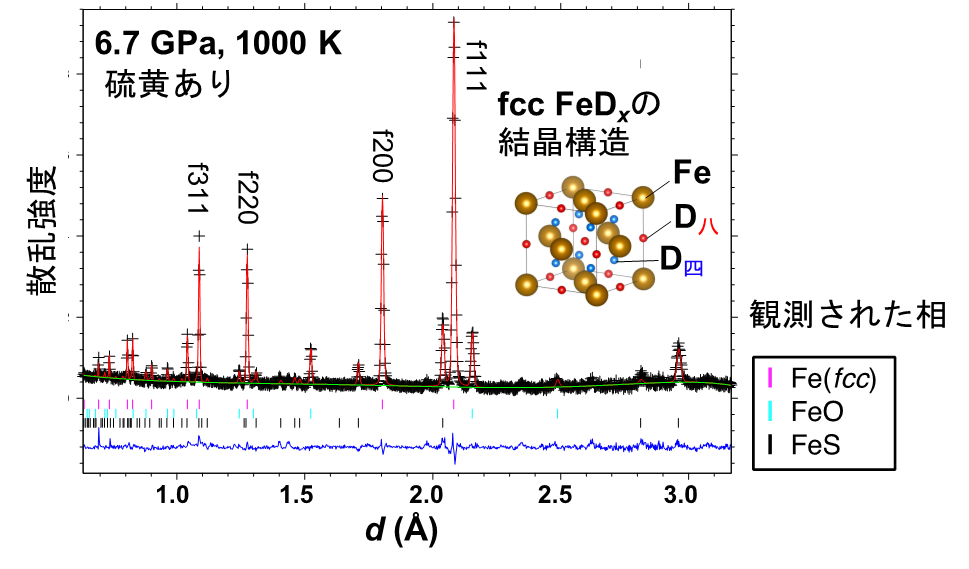
Figure 1: An example of the analysis of neutron diffraction data obtained from long-term measurements at high pressure and high temperature (6.7 GPa, 1000 K). In the high-temperature and high-pressure phase (fcc) of deuterated iron FeDx, deuterium was incorporated into the octahedral ( D8 ) and tetrahedral ( D4 ) sites in the crystal structure, but not into the coexisting iron sulfide FeS.

Figure 2: Relationship between the amount of deuterium incorporated into the high-pressure and high-temperature phases (fcc and hcp) of iron obtained by the analysis and temperature and pressure (the number attached to each data is the pressure value). A positive correlation was found for temperature and pressure, with the amount of deuterium decreasing in the sample with sulfur compared to the sample without sulfur. It is also expected that the solubility gap, which exists around x ~0.4, cannot be exceeded even at higher temperature and pressure.
Social Significance and Future Plans
In this study, we found that hydrogen and sulfur are both incorporated into solid iron as iron hydride and iron sulfide, respectively, in the presence of water. This result suggests that hydrogen may have preferentially dissolved into solid iron during the initial stage of primordial material accumulation on the primitive Earth, and that the coexistence of iron hydride and iron sulfide significantly lowered the melting point of iron, making it easier to melt at lower temperatures. It is possible that other light elements were gradually incorporated into the molten iron compounds in this manner, sinking into the Earth's core and ultimately forming the core (Figure 3). In order to solve the mystery of light elements in the Earth's core, it is necessary in the future to consider the contributions of multiple light elements simultaneously in order to clarify when and to what extent not only hydrogen and sulfur but also other candidate light elements were incorporated into iron, taking into account the origin and amount of water during the Earth's evolution.
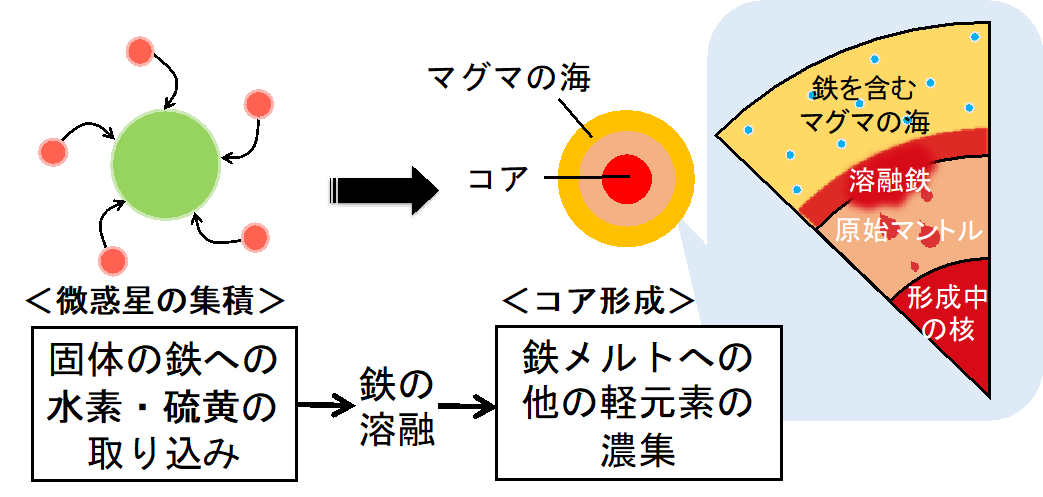
Figure 3: Scenario for the incorporation of light elements into iron during the formation of the primitive Earth. Hydrogen and sulfur were incorporated into solid iron during the accumulation of micrometeorites, and other light elements are thought to have been concentrated in the iron melt created by the lowering of the melting point to form the core. When and to what extent water was brought to Earth is the key to solving the mystery of the light elements in the core.
This research result was supported by Young Scientists' Research (Project No. 18K13630), New Academic Area Research (Project No. 15H05828), Basic Research S (Project No. 18H05224), Sumitomo Foundation Basic Science Research Grant, and KEK-Institute for Materials Structure Science Quantum Beam Science Research Grant. Improvement and preparation of the high-pressure cell and analysis of the recovered samples were conducted through the Collaborative Research Program of ISSP.
Journals
-
Journal name Scientific Reports Title of paper Behavior of light elements in iron-silicate-water-sulfur system during early Earth's evolution Author(s) Riko Iizuka-Oku*, Hirotada Gotou, Chikara Shito, Ko Fukuyama, Yuichiro Mori, Takanori Hattori, Asami Sano-Furukawa, Ken-ichi Funakoshi, Hiroyuki Kagi DOI Number 10.1038/s41598-021-91801-3 URL
Terminology
1 Neutron diffraction
Neutron diffraction is a technique for analyzing the crystal structure (the ordered arrangement of atoms in a solid state material) and magnetic structure of a material by using the physical phenomenon of diffraction when neutrons strike the material. This allows us to determine where and how much of a light element with a small number of electrons is present in the crystal structure of a material and to "see" it directly. For this reason, neutron diffraction, with its large scattering intensity even for light elements such as hydrogen, can provide complementary information to X-ray diffraction. In actual neutron diffraction experiments, samples in which light hydrogen (H) is replaced by deuterium (D) are used. This is to prevent H from causing strong incoherent scattering to neutrons and giving a large background, which makes precise structural analysis of the obtained diffraction patterns difficult. ↑up
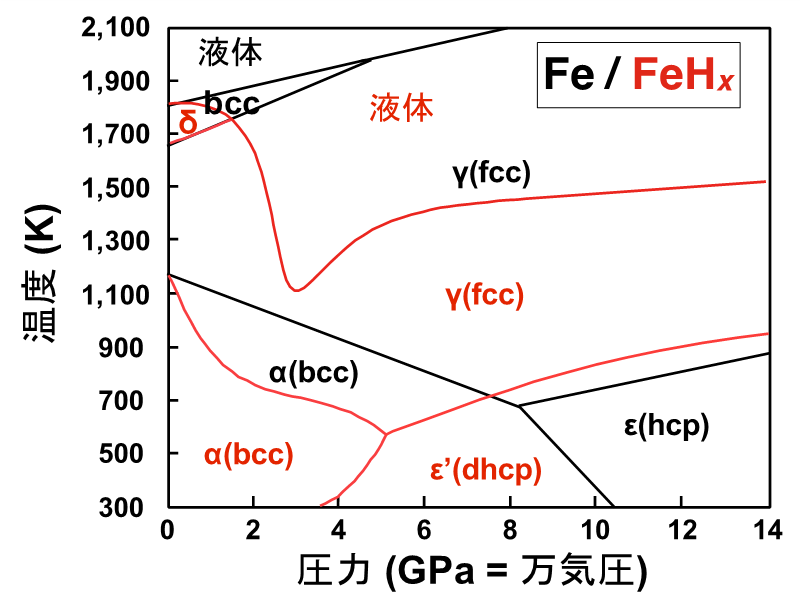
Note 2: Hydrogenation of iron (formation of iron hydride )
It is known that iron dissolves a large amount of hydrogen at pressures above 3.5 GPa (1 GPa is approximately 10,000 atm) to form iron hydride (FeHx ) and that the phase boundary changes and the melting point drops significantly. Iron hydride is a stable state in iron crystals in which hydrogen penetrates between iron atoms arranged in regular layers. It is known to change to various structures (phases) depending on temperature and pressure conditions (i.e., phase transitions), and the amount of hydrogen contained also depends on the temperature and pressure. As shown in the figure below, the fcc(γ) phase with a face-centered cubic structure and the hcp(Є) phase with a hexagonal close-packed structure, which are high temperature and high pressure phases of iron, undergo hydrogenation. The iron hydride dhcp(Є') phase (x = 1.0), in which an equimolar amount of hydrogen is incorporated with iron, is also known. In-situ observation at high pressure and high temperature is essential for the measurement of iron hydride because the bcc(α) structure is formed and hydrogen is exhaled as the pressure temperature is lowered. ↑up
Note 3 Core-mantle differentiation
The present-day Earth has a layered structure from the surface to the center: crust - upper mantle - lower mantle - outer core - inner core. The primitive Earth, on the other hand, was in an undifferentiated state with primordial materials still accumulated, and the mantle and core (core) were not separated. Later, the core was formed by the gravitational sedimentation of iron and separated from the mantle, which is composed mainly of light silicates, to form the present layered structure. ↑up
Note 4: Ultra-high pressure neutron diffractometer PLANET
The PLANET is an ultra high-pressure neutron diffractometer installed at the Materials and Life Science Experimental Facility (MLF) of the Japan Proton Accelerator Research Complex (J-PARC) in Tokai-mura, Ibaraki Prefecture, and enables in situ neutron observation under high temperature and high pressure conditions equivalent to those found deep within the Earth by combining pulsed neutron beams and large high-pressure devices. experiments can be performed under high-pressure conditions at about 360 GPa at the center of the earth, while neutron experiments require large sample volumes and are still limited in the pressure range available for such experiments. ↑up
Note 5 Rietveld method
This is one of the analysis methods to obtain structural information from diffraction patterns measured in powder X-ray diffraction and neutron diffraction experiments. Specifically, it involves refining parameters related to crystal structure and peak shape by comparing virtual diffraction patterns obtained by modeling parameters related to crystal structure (crystal lattice size and atom positions) with diffraction patterns actually obtained as experimental data. ↑up