DATE2021.03.27 #Press Releases
Elucidating why antimalarial drugs lose efficacy
Disclaimer: machine translated by DeepL which may contain errors.
~Expectations for the development of drugs that are unaffected by pathogen mutation
Tomohisa Kitagawa (2nd year, Master's course, Department of Biological Sciences)
Atsushi Matsumoto (Department of Biological Sciences, 3rd year of Doctoral program)
Ichiro Terashima (Professor, Department of Biological Sciences)
Yukifumi Uenono (Assistant Professor, Department of Biological Sciences)
Key points of the presentation
- We have elucidated the cause of the loss of efficacy of the antimalarial drugs quinacrine and chloroquine.
- We have shown for the first time the mechanism by which antimalarial drugs act on lipid membranes and the mechanism by which drug resistance is caused by changes in health (pH) conditions on the patient side.
- It is expected to be useful for the development of antibacterial and antiviral drugs that are not affected by mutations on the pathogen side.
Summary of Presentation
Antimalarial drugs are known to have multifaceted pharmacological actions such as antibacterial, antiviral, and immunosuppressive effects in addition to antiprotozoal activity, but the mechanisms of their actions and resistance remain unclear. A research group led by graduate students Tomohisa Kitagawa (at the time of the research), Atsushi Matsumoto (at the time of the research), Ichiro Terashima (Professor), and Koji Uesono (Assistant Professor) of the Graduate School of Science, The University of Tokyo, has revealed for the first time the mechanism by which antimalarial drugs change into a basic amphiphilic drug (CAD) structure (Note 1) when pH increases, localize on the yeast lipid membrane, and inhibit monosaccharide transporter function to exhibit antibacterial activity. This is the first clarification of this mechanism. Based on this mechanism, a decrease in the proportion of the CAD structure with a slight decrease in pH would cause antimalarial drugs to lose efficacy and become apparently resistant. These results provide a new perspective on the mechanism of drug resistance, and are expected to provide a method for selecting and searching for appropriate drugs, as well as for the development of antibacterial and antiviral drugs that are not affected by membrane protein mutations on the pathogen side.
Announcement
Some antimalarial drugs have been used as clinical agents not only for their antimalarial activity but also for their immunosuppressive effects. At the cellular level, antimicrobial and antiviral actions against various microorganisms as well as anticancer effects are also known, but it is not known why they have such multifaceted pharmacological actions. In addition, many of these cellular effects cannot be confirmed clinically, as evidenced by the ambiguous clinical effects of the antimalarial drug chloroquine on the new coronavirus. This discrepancy between cellular and clinical effects is one of the reasons for the enormous cost of drug development, not only for antimalarial drugs. Furthermore, chloroquine is still effective against three species of Plasmodium falciparum: oval, three-day fever, and four-day fever malaria parasites, but not against tropical fever malaria parasites, which have the highest mortality rate. For this reason, researchers around the world are focusing on the Plasmodium side of the factor, but it has yet to be elucidated.
In this study, we decided to elucidate the relationship between the biological effects of antimalarials and pH by focusing on the structural changes of antimalarials in response to pH, rather than from the conventional perspective of the Plasmodium falciparum side. Therefore, we focused on the antibacterial effects of antimalarials and analyzed them using budding yeast. The structure of quinacrine (QC), which was first developed in 1932 as an artificially synthesized malaria drug, is very similar to that of chlorpromazine (CPZ), an antipsychotic (Figure 1).
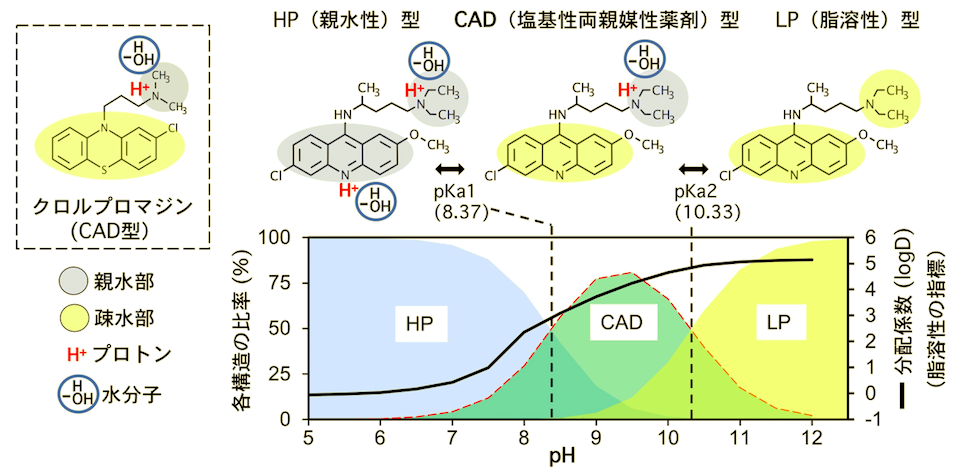
Figure 1: Structural changes of the antimalarial drug quinacrine in response to pH.
However, QC accumulates in the acidic vacuole of yeast, whereas CPZ localizes to lipid membranes. The reason for this difference in localization was investigated from the structural side: CPZ is a basic amphiphilic drug (CAD) structure with a hydrophobic moiety and a positively charged hydrophilic moiety, so it is thought to localize to lipid membranes with the same amphiphilic structure. We suspected that a similar CAD structure might be present in QC, so we used computational chemistry to estimate and analyze the structural changes, and found that QC changes its structure with different physical properties, from hydrophilic (HP), CAD, to lipophilic (LP) forms, by deprotonating nitrogen in response to increasing pH (Figure 1) The antibacterial action of QC on yeast is, exponentially enhanced (approximately 600-fold) as the CAD type increased from pH 5 to pH 8, suggesting that the CAD type exhibits antimicrobial activity. The CAD-type QC also localizes to the yeast lipid membrane and, like CPZ, induces sugar starvation by inhibiting the function of monosaccharide transporters at sites other than substrate recognition, and at high concentrations disrupts the membrane itself. Thus, it appears that CAD-type QC, localized nonspecifically along the orientation of lipid membranes, exerts multifaceted pharmacological effects by inhibiting the functions of various membrane proteins (Figure 2).
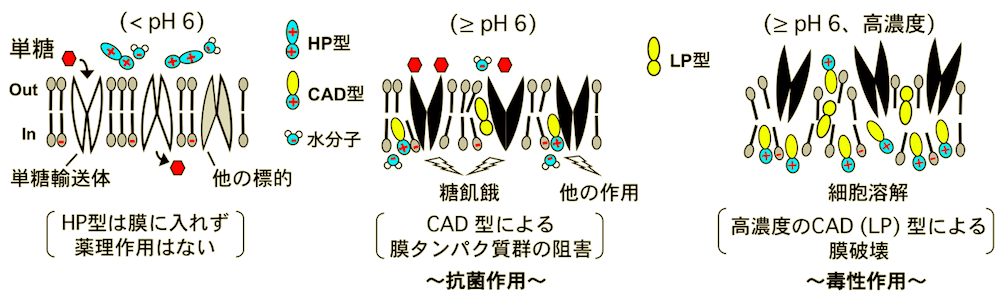
Figure 2: Mechanism of action and toxicity of the antimalarial drug quinacrine.
Since the increase in CAD type and exponential enhancement of antibacterial activity with increasing pH was also observed with chloroquine, we conclude that the CAD structure is important for the pharmacological action of these antimalarials. This conversely indicates that a slight decrease in CAD structure from a normal blood pH of 7.4 results in a significant decrease in drug efficacy. In other words, because tropical fever malaria and COVID-19 patients suffer from concomitant fluid acidification, these antimalarials lose efficacy with the degree of acidification and, in severe cases, lose efficacy. This may be the reason why the cellular level drug action evaluated at a single pH (7.4) does not match the clinical level of complex pH. Furthermore, this study revealed that the pH range in which the CAD type is formed differs among drug species (Figure 3). Based on this, selection of a drug that forms the CAD type at the pH of the affected area may be effective.
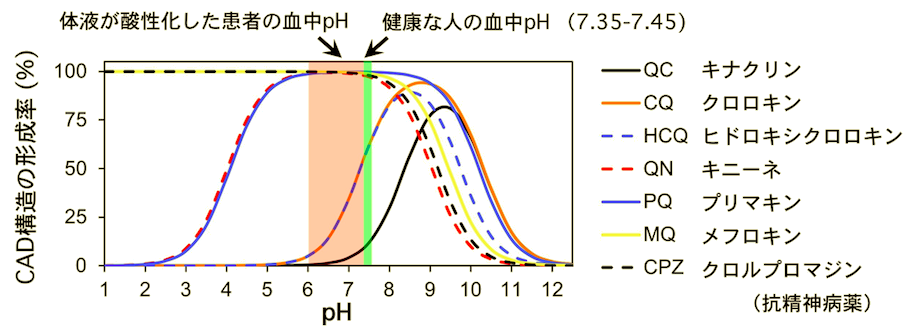
Figure 3: pH distribution of CAD structures of various antimalarial drugs
Based on the results of this study, drug resistance is considered to be caused not only by the emergence of resistant bacteria (cells), but also by changes in the health status (pH) on the patient's side, depending on the drug. Therefore, in the case of infectious diseases, it is necessary to select and search for drugs based on the understanding of the pH state of the patient and the infection site. For example, if we dare to administer an antimalarial drug to a COVID-19 severely ill patient whose body fluids are acidified due to pneumonia, quinine or primaquine, which do not decrease CAD structure with decreasing pH, may be appropriate instead of chloroquine-based drugs (Figure 3). Such pH-dependent changes in structure and physical properties can be observed not only in antimalarials but also in a variety of other compounds, which may provide a new perspective on the drug resistance mechanism. In addition, since CAD-based drugs target lipid membranes (Fig. 2), if designed well, they may become excellent antibacterial and antiviral drugs that are not affected by mutations in membrane proteins on the pathogen side.
Journals
-
Journal name Journal of Medicinal Chemistry Title of paper Antimalarial quinacrine and chloroquine lose their activity by decreasing cationic amphiphilic structure with a slight decrease in pH Author(s) Tomohisa Kitagawa, Atsushi Matsumoto, Ichiro Terashima, and Yukifumi Uesono* (author) DOI Number URL https://doi.org/10.1021/acs.jmedchem.0c02056
Terminology
1 Amphiphilic structure
A general term for compounds that have a hydrophilic part in one molecule that is easily compatible with water molecules and a hydrophobic part that is difficult to be compatible with water molecules. Known compounds include alcohols, surfactants, and lipid membranes of living organisms. ↑up


