DATE2021.04.07 #Press Releases
Solving a 40-Year-Old Problem in the Mannich Reaction
Disclaimer: machine translated by DeepL which may contain errors.
Yasuhiro Yamashita, Associate Professor, Department of Chemistry
Osamu Kobayashi, Professor, Department of Chemistry
Key points of the presentation
- In the Mannich reaction (Note 1 ), which is one of the most fundamental and important organic reactions, we have developed a catalytic asymmetric Mannich reaction directly using amides and esters as raw materials, which had been pending for 40 years, and succeeded in efficiently obtaining optically active β-amino acid derivatives (Note 2 ).
- By using a new asymmetric strong base hybrid catalyst system (Note 5) consisting of potassium hexamethyldisilazide (Note 3 ) and potassium salt of optically active bisoxazoline (Note 4), which has higher activity than conventional asymmetric catalysts, the desired reaction proceeds in high yield and high chiral selectivity is achieved.
- The results of this study are expected not only to provide an efficient synthetic method for optically active β-amino acid derivatives, but also to open up various catalytic asymmetric synthesis (Note 6) methods using less reactive raw materials.
Summary of Presentations
Catalytic asymmetric synthesis, which can efficiently and selectively obtain optically active compounds (Note 7), is one of the most important methods for the efficient synthesis of fine chemicals such as pharmaceuticals. In this study, Professor Osamu Kobayashi and his group at the Graduate School of Science, The University of Tokyo, have developed a new highly active catalytic system for the catalytic asymmetric Mannich reaction that can synthesize optically active β-amino acid derivatives, which are raw materials for pharmaceuticals, and have succeeded in achieving the production of amides and esters, which have been a 40-year-long concern in organic synthetic chemistry. The new catalytic system enables the use of amides and esters, which have very poor reactivity, as raw materials without prior activation, which has been a 40-year-old concern in organic synthetic chemistry. The use of these raw materials has been impossible with previously reported catalysts due to their low activity.
Instead of using highly reactive raw materials such as aldehydes and ketones, which have been commonly used in this reaction, we investigated the asymmetric Mannich reaction using amides and esters, which are readily available but difficult to use as raw materials due to their low reactivity, and found that the following compounds, which are very basic, can be used in the reaction. We found that by using a catalyst system prepared from potassium hexamethyldisilazide (KHMDS), which is a very basic compound, and optically active bisoxazoline, the desired reaction proceeds with high asymmetric selectivity (Note 8). It was also found that a catalyst preparation method different from the conventional method, in which an excess amount of KHMDS was used for bisoxazoline, was important for the construction of an effective catalyst species, and a new catalyst system, an asymmetric strong base hybrid catalyst system, was generated and found to be functional. This research is academically very important in that it is the first realization of catalytic asymmetric Mannich reactions using as-is amides and esters, which have been a long-standing concern in synthetic organic chemistry, as raw materials with very poor reactivity.
The research results were published in the online bulletin of the Journal of the American Chemical Society on April 57 (Japan Standard Time). This work was supported by the Japan Agency for Medical Research and Development (AMED) and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) under the Grant-in-Aid for Scientific Research on Innovative Areas, "Hybrid Catalysts" (JP 17H06448).
Announcement Details
Background of Research
Fine chemicals such as pharmaceuticals are indispensable for maintaining the welfare of mankind, and their prompt supply is strongly desired by society. For this reason, organic synthesis, which produces fine chemicals from natural resources such as petroleum, is now considered important. While many chemical industries have been producing and supplying various products to the market through organic synthesis, the byproduct waste from the production of such products has become a social issue because of its significant impact on the global environment. In particular, the release of carbon dioxide from the use of fossil fuels and the generation of waste containing toxic metals are known to cause global warming and environmental pollution, and major improvements are needed. As a means of solving such problems in organic synthesis, it is important to use "catalysts" that can carry out chemical reactions in an energy-efficient manner and generate less waste. On the other hand, chiral points often exist in the structure of fine chemicals such as pharmaceuticals. However, efficient and stereoselective construction of such asymmetric points is difficult and has been done with a lot of waste as a byproduct. In response to this problem, various catalytic asymmetric reactions using highly active asymmetric catalysts have been developed and applied in the chemical industry as a highly efficient and stereoselective synthetic method for compounds with chiral points, as exemplified by the Nobel Prize in Chemistry awarded to Ryoji Noyori et al. in 2001.
β-amino acid derivatives are important organic compounds with nitrogen atoms that are often used in pharmaceutical synthesis, and the development of efficient synthetic methods is required (Fig. 1 left). In particular, the asymmetric Mannich reaction, one of the methods for synthesizing optically active β-amino acid derivatives, is an efficient method to form carbon-carbon bonds between an imine, a compound with a carbon-nitrogen double bond, and a carbonyl compound, and to simultaneously form an asymmetric Mannich reaction between an imine and an amino acid. The asymmetric Mannich reaction is a fundamental and important reaction that can simultaneously construct a chiral point at the root of an amino group in a highly stereoselective manner. Since the Mannich reaction method using pre-activated carbonyl compounds was reported about 40 years ago, there have been many studies on this reaction, including the development of catalytic asymmetric reactions, but there was a major problem of low atomic efficiency (Note 9) due to the need for many additional reactants for the pre-activation. However, this requires a large number of additional reactants for pre-activation, which results in low atomic efficiency. Therefore, the development of asymmetric Mannich reactions that use carbonyl compounds as raw materials without prior activation has been strongly desired, but while many reactions using highly reactive malonic acid esters, aldehydes, ketones, etc. as raw materials have been reported, &beta However, while there have been many reports of reactions using highly reactive malonate esters, aldehydes, and ketones as raw materials, asymmetric Mannich reactions using readily available amides and esters as raw materials that can directly synthesize &beta-amino acid derivatives have been very difficult to achieve with conventional catalysts (Figure 1, right).
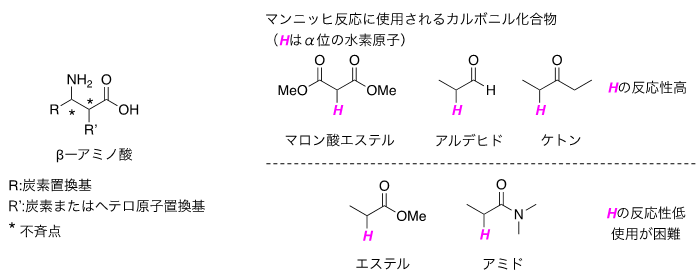
Figure 1: β-amino acids and carbonyl compounds as reaction raw materials
Details of Research
We developed asymmetric base-catalyzed reactions using imines with substituents on the nitrogen atom and commercially available N,N-dimethylpropionamide as raw materials, because N,N-dimethylpropionamide has a very low acidity of hydrogen atoms on the reaction point and deprotonation (Note 10) reaction is not possible using commonly used base catalysts. (10) reaction to generate anionic active species was difficult. In order to activate such low reactivity raw materials, potassium hexamethyldisilazide (KHMDS), a potassium strong base with stronger reactivity, was used as a catalyst, and in order to realize a catalytic reaction, an electron-rich aromatic ring was introduced as a protecting group on the nitrogen atom of imine. The catalytic reaction was carried out. We investigated various asymmetric ligands for asymmetric modification of potassium base catalysts and found that optically active crown ethers (Note 11), which have been frequently used, rarely work, while optically active bisoxazolines, which are commonly used as asymmetric ligands for a variety of metals, work effectively. We have discovered that the optically active bisoxazoline, a commonly used chiral ligand for various metals, works effectively. Furthermore, it was found that the use of an excess amount of KHMDS over bisoxazoline was important for the construction of an effective catalytic species. In the conventional method of constructing optically active potassium strong base catalysts, it is important to use approximately the same amount of potassium strong base catalyst for the asymmetric ligand, but the main feature of the new catalyst is that an excess amount of potassium strong base is required for the asymmetric ligand. Reactions with various imines, amides, and esters under optimal conditions revealed that high asymmetric selectivity was achieved (Fig. 2). The method was also used to efficiently synthesize SCH-48462, a β-lactam compound with cholesterol absorption inhibitory activity.
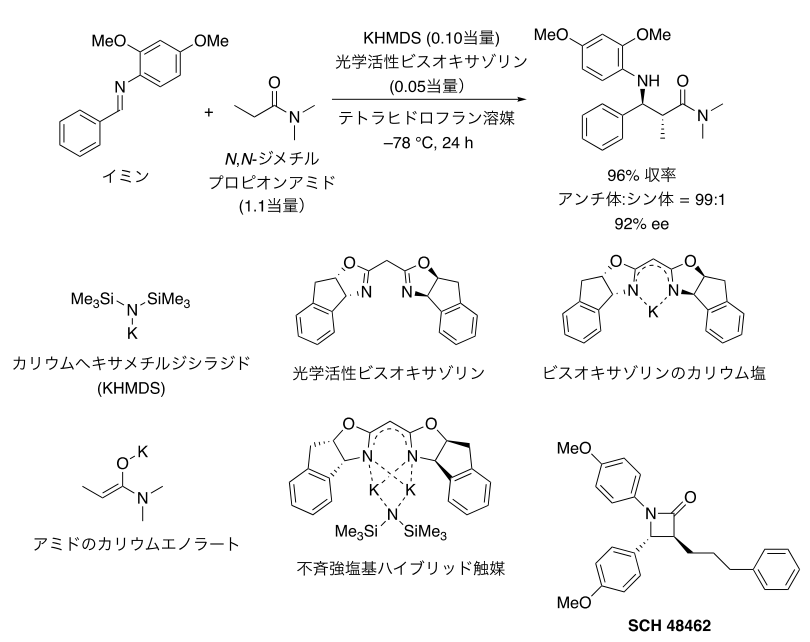
Figure 2: Catalytic asymmetric Mannich reaction using poorly reactive amides directly
This catalytic system was different from conventional strong base catalysts because it required the use of more than double the amount of potassium strong base relative to bisoxazoline. The structure of the catalyst was elucidated by nuclear magnetic resonance (NMR) spectroscopy and computational chemistry, and it was found that bisoxazoline is deprotonated by KHMDS to form its potassium salt, which then interacts with another KHMDS as a chiral ligand to form an asymmetric strong-base hybrid catalytic system. In this reaction, the new catalytic system deprotonates the raw amide to generate the potassium enolate of the amide, (Note 12 ), which reacts with the imine to form the target product. Furthermore, we attempted to elucidate its detailed structure by computational chemistry, and suggested that it may have a unique structure (Figure 3), in which two potassium ions fit equally between two nitrogen atoms of bisoxazoline.
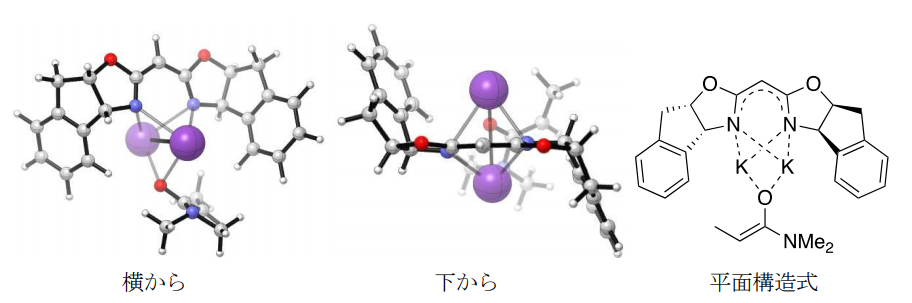
Figure 3: Structure of hybrid-type reactive active species consisting of potassium salt of bisoxazoline and potassium enolate, as shown by computational chemistry.
This research is academically very important in that it is the first realization of a catalytic asymmetric Mannich reaction that uses poorly reactive amides as raw material carbonyl compounds, which has been a longstanding issue in synthetic organic chemistry and has been extremely difficult to achieve.
Future Prospects
The results obtained in this study are expected to lead to the development of various catalytic asymmetric Mannich reactions using raw materials that are abundant on the earth with very low acidity of hydrogen atoms existing on the reaction point.
Journal
-
Journal name Journal of the American Chemical Society Title of paper Chiral Metal Salts as Ligands for Catalytic Asymmetric Mannich Reactions with Simple Amides Author(s) Yasuhiro Yamashita*, Aika Noguchi, Seiya Fushimi, Miho Hatanaka and Shū Kobayashi* (author) DOI Number 10.1021/jacs.0c13317 URL https://pubs.acs.org/doi/10.1021/jacs.0c13317
Terminology
Note 1 Mannich reaction
A reaction that occurs between a compound called imine, which has a carbon-nitrogen double bond, and a carbonyl compound such as an aldehyde or ketone. The carbon atom in the double bond of imine reacts with the carbon atom next to the carbonyl group ( α-position ) of the carbonyl compound to form a carbon-carbon single bond, while the carbon-nitrogen double bond of imine changes to a single bond. At the same time, the carbon-nitrogen double bond of imine is converted to a single bond. In this process, a chiral point is created on the reacted carbon. The reaction to selectively obtain one of the mirror isomers by controlling the position of the chiral point is called the asymmetric Mannich reaction. ↑up
Note 2 β-amino acid derivatives
A derivative of an amino acid in which the space between the amino group and the carbonyl group is one carbon atom longer than the commonly known amino acid ( α-amino acid ). In this case, β-aminoamide andβ-aminoester are referred to here. ↑ (α-amino acid)
Note 3 Potassium hexamethyldisilazide (KHMDS )
Strong base with potassium ion. It has a potassium-nitrogen bond and two silicon substituents on the nitrogen atom. The nitrogen atom is a strong base. ↑up
Note 4 Bisoxazoline
A structure in which two heterocycles called oxazolines are connected via a single carbon atom. The nitrogen atom on the hetero ring can strongly interact with metal atoms. ↑up
Note 5 Hybrid catalyst system
A catalytic system in which the functions of multiple catalysts (or catalytic sites) with independent functions are utilized in a concerted manner. In this case, it refers to a catalytic system in which an optically active metal salt catalyst and a strong base catalyst are hybridized. ↑up
Note 6 Catalytic asymmetric synthesis
A method to synthesize a theoretically infinite number of one-way mirror-image isomers using only a few chiral sources (chiral ligands). Ryoji Noyori et al. won the Nobel Prize in Chemistry in 2001. A mirror-image isomer is a molecule with a molecular structure in which the mirrored image has a non-overlapping relationship with the original image (right-handed and left-handed). ↑up
Note 7 Optically active compounds
A compound in which one of the two mirror-image isomers of a compound with a chiral point is present in excess of the other isomer. ↑up
Note 8 Asymmetric selectivity
A value indicating how preferentially (selectively) one of the mirror isomers is obtained. It is expressed in terms of optical yield (enantiomeric excess, ee(%)). When the ratio of both mirror isomers is 99:1, the ratio is 98% ee. ↑ee(%)
Note 9 Atomic efficiency
A value indicating how much of the atoms in the raw materials used in a reaction are included in the product. The higher the ratio of atoms in the raw material incorporated into the product, the higher the atomic efficiency. Atoms that are not incorporated into the product become waste. ↑up
Note 10: Deprotonation
A reaction in which the hydrogen atoms of a carbon-hydrogen bond are removed as protons ( H+). The remaining carbon atoms become anionic (negatively charged). ↑up
Note 11 Crown ether
A cyclic ether compound in which multiple oxygen atoms are regularly arranged in the structure. ↑up
Note 12 Potassium enolate
A highly reactive chemical species formed by deprotonation of a hydrogen atom on the carbon atom adjacent to the carbonyl group ( α-position ) of a carbonyl compound by a potassium base. The negative charge produced is mainly on the oxygen atom derived from the carbonyl group, and the bond between the carbonyl carbon and the carbon atom at the α-position takes on a double bonding character. Potassium ions interact predominantly with oxygen atoms. ↑


