DATE2024.02.13 #Press Releases
The Mystery of the Earth! What is the secret of metals in the soil?
Disclaimer: machine translated by DeepL which may contain errors.
-Molecular Scale Simulations and Observations Reveal the Properties of Soil
Japan Atomic Energy Agency
School of Science, The University of Tokyo
Institute for Radiation Sciences, Osaka University
Isotope Science Center The University of Tokyo
Summary of Presentations
A research group led by Akiko Yamaguchi, Research Fellow, and Masahiko Okumura, Senior chief, at the Centre for Computational Science & e-Systems of JAEA and Prof Yoshio Takahashi at the University of Tokyo has found a tendency in the molecular-level adsorption of metal ions by clay minerals for ions that are less soluble in water and have larger ionic radii to be strongly adsorbed by clay minerals and shown that this tendency is also true in natural soil environments.
For geological disposal of radioactive waste and decontamination of radioactive materials, it is necessary to elucidate the behavior of radioactive elements in soil as metal ions. Since metal ions are often adsorbed on clay minerals, which are abundant in soil, it is necessary to understand adsorption reactions of metal ions on clay minerals in order to understand and predict their behavior. This phenomenon is important in resource mining, environmental chemistry, agriculture, and even astrochemistry, since many metal ions, including rare earth elements (REEs), are adsorbed on clay minerals in fields other than nuclear energy.
However, adsorption reactions between metal ions and clay minerals are complex. For example, some metal ions including rare earths are easily desorbed after adsorption on clay minerals, while metal ions such as cesium are not easily desorbed once adsorbed on clay minerals. It has not been clarified why such different phenomena are observed for different types of metal ions.
To elucidate the cause of this phenomenon, this study combined molecular-level experiments using synchrotron radiation facilities and high-precision simulations using a supercomputer to elucidate the structure of metal ions adsorbed on clay minerals at the molecular level. These results revealed a tendency for clay minerals to strongly adsorb ions that are less soluble in water and have a large ionic radius. Furthermore, analysis of soil samples collected by boring confirmed that various metal ions were distributed in accordance with the trends obtained from experiments and simulations. Therefore, the trends obtained in this study can be applied to understanding and estimating the movement of metal ions in environmental soils.
Through this study, we have elucidated the behavior of metal ions, including radioactive elements, in soil. We have also obtained important knowledge for understanding the movement of metal ions on the earth and, moreover, on planets other than the earth. In particular, this research has elucidated radium, a radioactive element whose chemical properties remained unknown for more than 100 years after its discovery because of its difficult handling. Radium has attracted worldwide attention in recent years as a raw material for actinium, which is effective in cancer therapy. This discovery shows that radium with a large ionic radius is strongly adsorbed on clay minerals, which is expected to be useful in future research for the recovery of radium. In addition to radioactive elements, this discovery is expected to lead to the solution of other socially important issues such as efficient exploration of rare earth deposits, efficient agriculture, and estimation of the environments of planets in the solar system (Mars, Lyugu, etc.).
The results of this research were published in Elsevier's Journal of Colloid and Interface Science on February 1 (Japan time).
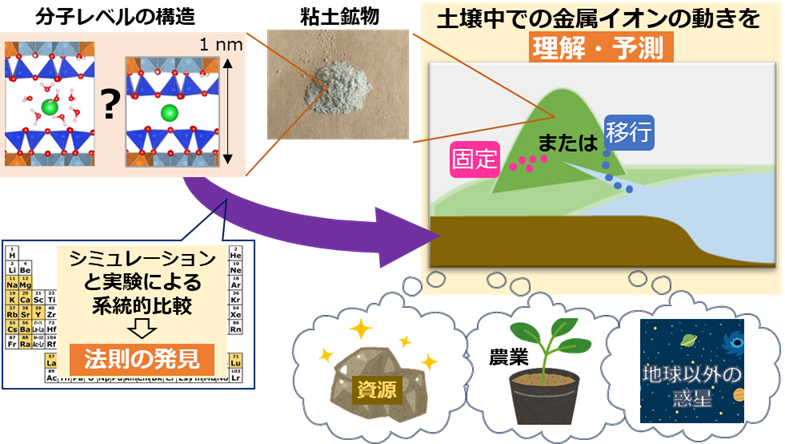
Figure: Overview of this research
For more information, please visit the website of Japan Atomic Energy Agency (JAEA).
Journal
-
Journal name Journal of Colloid and Interface ScienceTitle of paper


