DATE2023.12.21 #Press Releases
Teaching an old dog new tricks in the supramolecular chemistry, the chemistry of equilibrium
A study addresses the stoichiometry of the chemical components involved in equilibrium.
Dec 21, 2023
As in cooking recipes, the proportion of reacting components is important in chemistry. The ratio of components is called stoichiometry, and determining the stoichiometry is the first step in understanding chemical interactions. Although statistical and information-theoretic measures have recently been introduced to estimate the stoichiometry for various equilibria, a study led by Hiroyuki Isobe’s team from the University of Tokyo questions the validity of the known measures of stoichiometry.
The traditional methods of determining stoichiometries include ‘Job plots’ of chemical titration, which has recently been replaced by modern methods adopting statistical and information-theoretic measures. However, these methods were found to give false answers to difficult, modern cases in supramolecular chemistry. The supramolecular complexes are assembled via equilibrium controlled by weak non-covalent interactions, and it is not easy to decide the stoichiometry of the components in equilibrium.
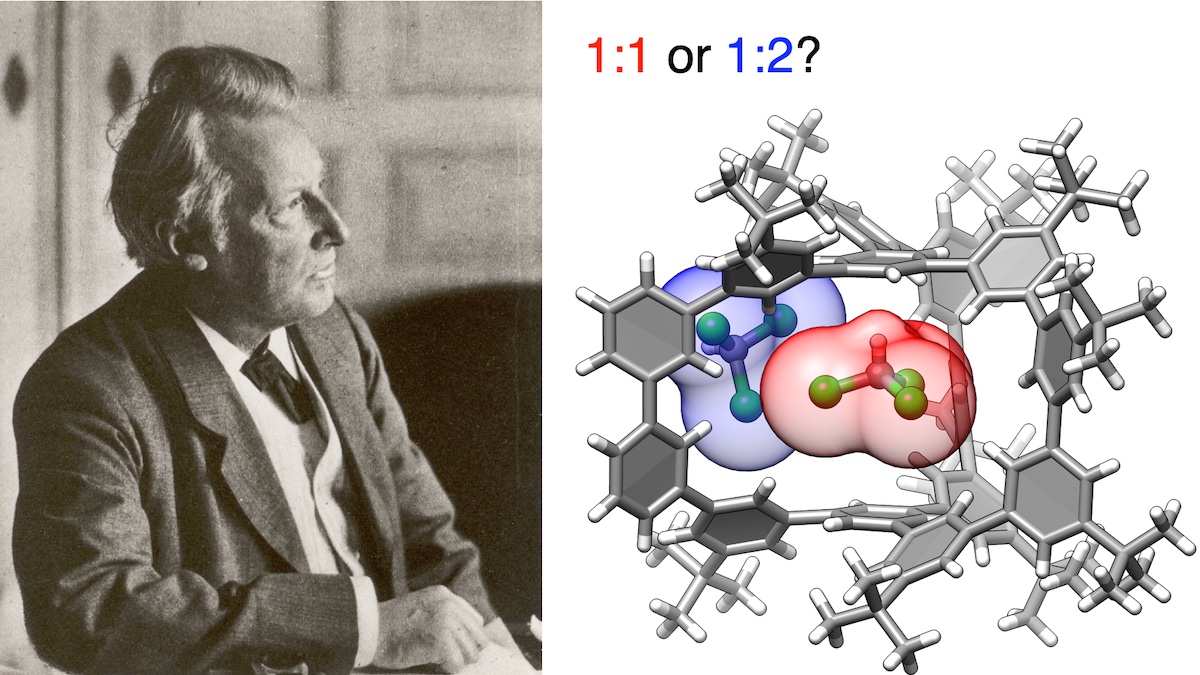
Figure. Left: Jacobus Henricus van ’t Hoff, a Dutch chemist. Right: A supramolecular complex with a hydrocarbon cage and chloroform molecules interacting via weak CH-π hydrogen bonds. The van ’t Hoff analyses (which won the first Nobel Prize in Chemistry) allowed the team to validate the stoichiometries of supramolecular complexes.
In this study, the research team offered a new method to determine correct stoichiometry for equilibrium to form supramolecular complexes. For that, the team adopted so-called van ’t Hoff plots (originally invented in 1884 to clarify thermodynamic parameters) to now determine the correct stoichiometry. The study determined the number of chloroform molecules the hydrocarbon cage can host—a 1:1 ratio being the correct answer that matched theoretical predictions (see Figure).
The findings will have an impact on various fields, including drug development and biological reactions. The paper appears in the journal Nature Communications.
For more details, please read the article:
Toshiya M. Fukunaga, Yuzuka Onaka, Takahide Kato, Koki Ikemoto and Hiroyuki Isobe. 2023. Stoichiometry validation of supramolecular complexes with a hydrocarbon cage host by van 't Hoff analyses. Nature Communications. DOI: 10.1038/s41467-023-43979-5.


