DATE2023.10.03 #Press Releases
Demonstration of ammonia concentration mechanism in an ancient deep-sea hydrothermal vent environment
Disclaimer: machine translated by DeepL which may contain errors.
-The secret to selective adsorption of ammonia lies in the electroreduction of iron sulfides.
Japan Agency for Marine-Earth Science and Technology
School of Science, The University of Tokyo
Summary of Presentation
Dr. Wataru Takahagi, Research Student (currently Postdoctoral Fellow, Rensselaer Polytechnic Institute), Dr. Ken Okada, Research Fellow, Dr. Ken Takai, Director, Dr. Norio Kitadai, Senior Staff, Associate Senior Staff, Dr. Yohei Matsui, Associate Senior Staff, Dr. Yohei Matsui, Associate Senior Staff, Dr. Yohei Matsui, Associate Senior Staff, Dr. Yohei Matsui, Associate Senior Staff, Dr. Yohei Matsui, Associate Senior Staff, and Dr. Shigeaki Ono, Director, Marine Earthquake and Volcano Research Center, all from the Ultra-Pioneering Research Division, Japan Agency for Marine-Earth Science and Technology (JAMSTEC), will present their research in the "Electrochemical Adsorption of Ammonia" project. Director Shigeaki Ono and his colleagues, in collaboration with Professor Yoshio Takahashi of the Graduate School of Science, The University of Tokyo, have clarified the mechanism of ammonia concentration in ancient deep-sea hydrothermal vent environments.
Deep-sea hydrothermal vent environments are one of the most promising candidates for the origin of life. Laboratory experiments simulating actual deep-sea hydrothermal vent environments have shown that biomolecules such as amino acids and nucleobases may have been generated nonbiologically. However, the formation of these organic nitrogen compounds requires high concentrations of ammonia. Although several geochemical processes have been proposed to supply ammonia around the vent ( as reported on June 21, 2019 ), it was unclear how the supplied ammonia was retained and concentrated.
In this study, Takahagi and his research group demonstrated that by electroreducing iron sulfides (mackinawite), which are ubiquitous in deep-sea hydrothermal vent environments, adsorption sites consisting of zero-valent iron atoms ( Fe0 ) can be generated in the layer structure, dramatically improving ammonia adsorption capacity (Figure). The reduction of mackinawite to Fe0 (FeS + 2H+ + 2e- → Fe0 + H2S ) proceeds below -0.6 V (vs. standard hydrogen electrode potential), and in a 48-hour experiment, up to >90% ammonia adsorption was achieved (initial concentration 1 mmol L-1 ) from an aqueous solution mimicking ancient seawater (1 mol L-1 NaCl, neutral pH).
The potential conditions (-0.6 V or lower) favorable for ammonia adsorption are also observed in existing hydrothermal vent environments, and are fully feasible on Earth. In addition, reducing (low-potential) hydrothermal fluids containing high concentrations of hydrogen have been universally erupted from the ancient ocean floor due to active rock-hydrothermal reactions beneath the seafloor (Figure). Taking into account the formation of ammonia from nitric and nitrite acids and the ability of mackinawite to promote reactions for amination, as shown in previous studies, the prehistoric deep-sea hydrothermal vent environment was a suitable site for the formation, concentration, and assimilation of ammonia as a result of common natural phenomena occurring at the site.
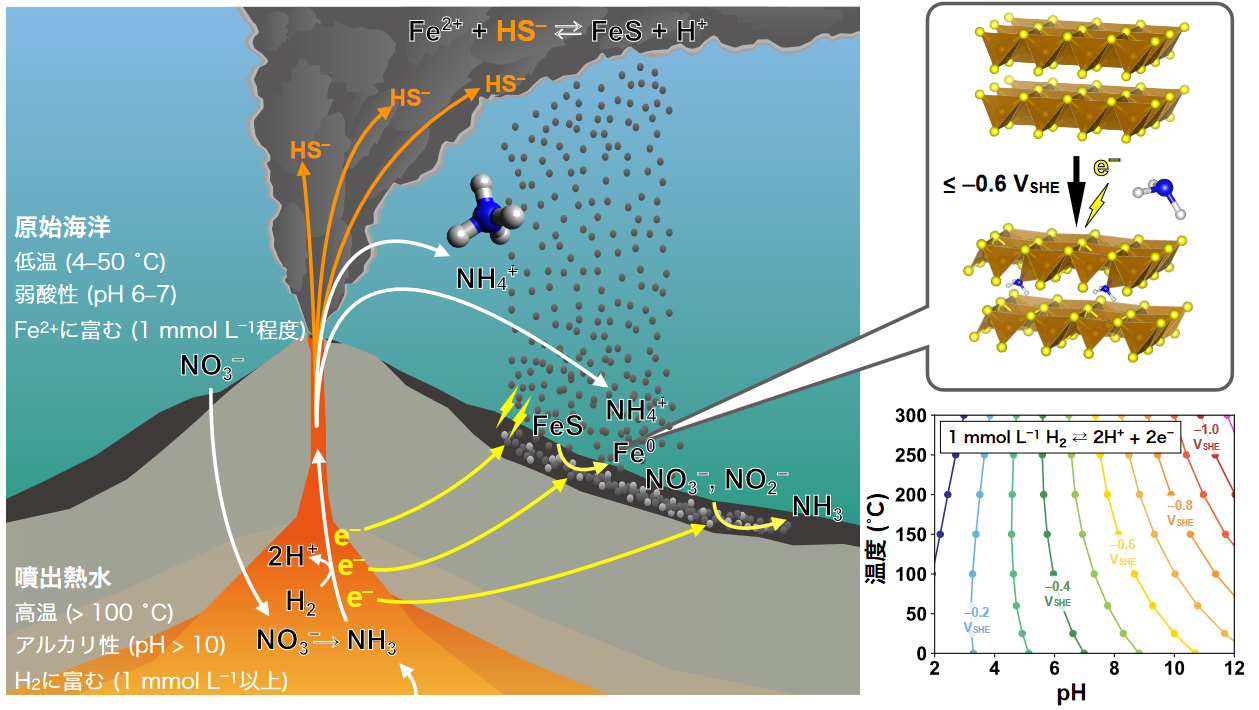
Figure: The process of ammonia production and assimilation in ancient deep-sea hydrothermal vent environments, as found in this study. Mackinawite is a mineral that is ubiquitous in deep-sea hydrothermal vent environments, and is produced by the reaction of hydrogen sulfide in hydrothermal water and iron ions in seawater ( Fe2+ + H2S → FeS + 2H+). A negative potential derived from the oxidation of reducing substances in the hot water (e.g., H2 → 2H+ + 2e-) is regularly applied in the vicinity of the vent, where the precipitation of mackinawite results in the electroreduction of iron ions in the layer structure to zero valence (upper right figure). The resulting zero-valent iron atoms function as adsorption sites for ammonia and can selectively adsorb ammonia even in concentrated salt water (1 mol/L NaCl). Electroreduction of Mackinawite proceeds below -0.6V. This potential can be achieved by oxidation of H2 to H+ in alkaline hydrothermal water (bottom right), and hydrothermal water with such quality is believed to have been widely erupted on the ocean floor in ancient times.
This work was supported by JSPS Grants-in-Aid for Scientific Research 19K04048, 19K15379, 20H00209, 21H04527, 22H05149, 22K03801 and 23K13211. The results will be published in "Proceedings of the National Academy of Science" on October 3 (JST).
For more information, please visit the website of the JAMSTEC.
Journal
-
Journal name Proceedings of the National Academy of ScienceTitle of paper Extreme accumulation of ammonia on electroreduced mackinawite: An abiotic ammonia storage mechanism in early ocean hydrothermal systems


