DATE2022.12.21 #Press Releases
Shaken or stirred: the key to faster crystal growth
Atomic resolution time-lapse images reveal how crystals grow in never-seen-before detail.
December 21, 2022
Stir a concentrated salt solution; you get fine crystals. For the first time, Japanese researchers reveal how crystals grow at atomic resolution using high-speed imaging. Their findings show why stirring a concentrated salt solution speeds up crystal growth. The study provides hitherto overlooked insights to precisely control crystal growth. The techniques used in the study can have various industrial applications.
“The crystal formation consists of two stages: nucleation and crystal growth. Last year we captured images of the first stage, and now we have succeeded in analyzing the second stage using a camera that is 10 times faster,” says Eiichi Nakamura, an University Professor at the University of Tokyo.
During nucleation, ions and molecules arrange themselves in a specific pattern to form a site for more ions to join. Then the crystal growth starts. To film the process in extreme detail, the researchers built two techniques over the past 15 years. The first technique involves a microscopic vessel called a water-dispersible conical carbon nanotube. NaCl (Sodium Chloride) crystals are grown in the microscopic vessel allowing for better visualization. The second technique uses a transmission electron microscopy called single-molecule atomic-resolution time-resolved electron microscopy (SMART-EM). It can resolve individual molecules and can take images of moving ion pairs (Na+, Cl-) at 300 frames per second using a state-of-the-art camera. Thanks to the two techniques, Prof. Nakamura and his team showed that the continuous supply of NaCl molecules and gentle low-frequency vibration of the vessel promotes the crystal growth stage (Figure 1).
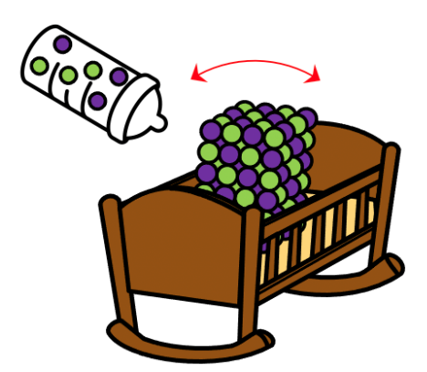
Figure 1 : Molecular supply and gentle vibration promote crystal growth.
The crystal growth begins with new ions gathering at the nucleation site. Soon after, a small island of NaCl ion pairs forms on top of the earlier-formed defect-free crystalline layers (see Figure 2 and Video 1). As the island is not fixed to the surface, the researchers call it a “Floating Island.” It assembles or disassembles and moves around with each vibration of the vessel. It grows larger or smaller until it loses kinetic energy and lands on the crystal layer triggering the rapid accumulation of a new crystalline layer. The Floating Islands occur rarely but are necessary for crystal growth. That is why stirring a saturated salt solution helps form Floating Islands that can speed up crystal growth. Without gentle vibrations, the crystals grow more slowly.
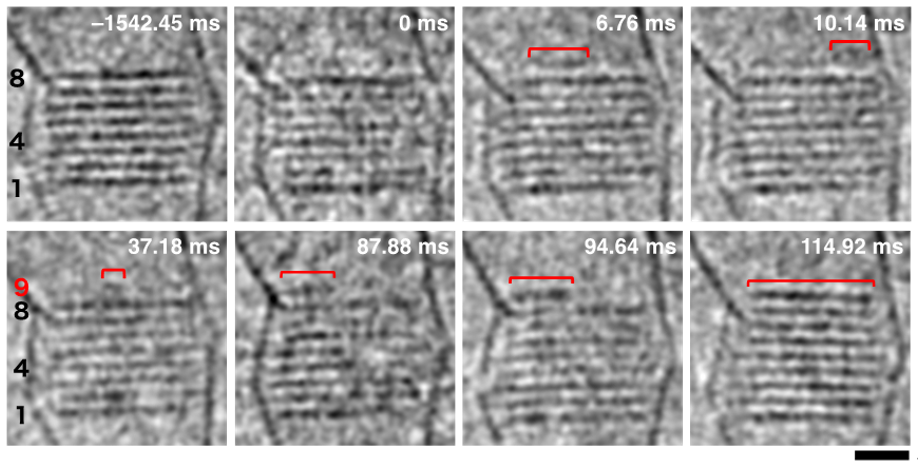
Figure 2 : Formation of Floating Island (red bars) on the layers of a NaCl nanocrystal. The numbers show the crystal layers; the 9th layer is where the Floating Island forms. ©2023 American Chemical Society.
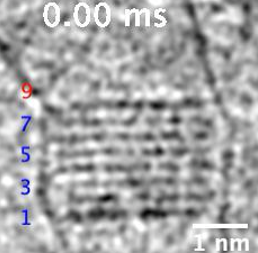
Video 1. Formation of Floating Island on a NaCl nanocrystal. See Figure 2 to locate the position of the floating island and how it changes in length as it grows. ©2023 American Chemical Society.
“The successful direct observation of the effects of vibration on crystal growth at atomic resolution has demonstrated that we can study how minute mechanical stimuli induce phenomena at the molecular level. This research method can help uncover the behavior of each molecule that generates the response of macroscopic molecular assemblies, which have been a black box until now. Based on the observations at the molecular level, new materials that exhibit the desired macroscopic response can also be developed. Moreover, we hope that SMART-EM images, which allow people to see the movement of atoms and molecules, will be a tool in chemistry education,” says Prof. Nakamura.
Publication details
Journal ACS Central Science Title Cinematographic Recording of a Metastable Floating Island in Two- and Three-Dimensional Crystal Growth Authors Masaya Sakakibara, Hiroki Nada, Takayuki Nakamuro and Eiichi NakamuraDOI
Podcast credits
Music: "Upbeat Forever" Kevin MacLeod (incompetech.com)
Sound effect: Zapslat.com
Podcast host: Dr. Ravindra Palavalli-Nettimi



