DATE2022.06.13 #FEATURES#The Rigakubu News
Mysteries in Science: Can we create new molecules using metal superatoms?
Tatsuya Tsukuda
(Professor, Department of Chemistry)
Atoms make up every material around us, which have diverse structures and properties depending on the elements, numbers, and bonding configurations of atoms. One of the ultimate goals of chemical research is to create materials with desired structures and properties by freely combining atoms that are on the periodic table. Our laboratory is interested in the development of a new building unit of material called a metal superatom, an ultrafine particle comprised of a certain number of metal atoms, and is working towards its systemization. This article will introduce our attempt at creating a new nanoscale molecule using metal superatoms.
To begin with, what are “superatoms”? Let me explain using a typical example, the icosahedral Au13 superatom, which consists of 13 gold (Au) atoms (Figure A). Electrons confined within the Au13 superatom are accommodated in quantized orbitals (called 1S, 1P, 1D, 2S, and so on, in the order starting from the most energetically stable), which are formed within the binding potential created by a cluster of 13 Au+ ions. Interestingly, these orbitals have similar shapes and the same numbers to the conventional atomic orbitals (such as 1s, 2s, 2p, 3s). Therefore, when a Au13 cluster has eight electrons, it becomes highly stable by forming a closed-shell electron configuration (1S)2(1P)6 resembling noble gas atoms. This hierarchical electronic structure is like that of atoms, which is why Au clusters are called superatoms. Furthermore, the electronic structures of Au superatoms exhibit periodicity with respect to the number of constituent atoms, meaning superatoms could be systematized in a periodic table of their own.
Metal superatoms can be synthesized as stable compounds by coating their surfaces with organic ligands, so naturally, chemists want to create molecules using superatoms. We call these quasi-molecules “superatomic molecules” to differentiate them from supramolecules, assemblies of molecules via non-covalent interaction. More specifically, the superatomic molecule refers to a structure created by sharing a part of the superatoms while retaining their original structure. For example, superatomic molecules such as Au25 and Au23, in which two icosahedral Au13 superatoms are bonded by sharing one or three Au atoms, have been reported (Figure B). These superatomic molecules, however, were obtained by chance as minor byproducts in the synthesis of superatoms.
Our lab, on the other hand, has developed a method for the targeted synthesis of various superatomic molecules by fusing the superatoms prepared in advance. We focused on the Au9 superatom as a starting material. The Au9 superatom has a closed-shell electron configuration of (1S)2(1P)4 because one of the three 1P orbitals is destabilized due to its oblate structure. By reacting with hydride (H–), the Au9 superatom is converted to the HAu9 superatom with a closed-shell electron configuration (1S)2(1P)6, which exhibits nucleophilic reactivity (Figure C). In fact, we successfully obtained a Au23 superatomic molecule with a bi-icosahedral structure in a high yield by mixing HAu9 superatoms with the Au13 superatoms. This finding suggests that the bonding of hydride can activate superatoms toward fusion to other metal superatoms. And sure enough, when we applied this method to the reaction between alloy superatoms HPd@Au8 and M@Au12 (M = Pd, Pt), we were able to successfully synthesize an unprecedented alloy superatomic molecule, PdMAu21 (Figure D).
How can we explain the bonding mechanism between superatoms? How many superatomic molecules with diverse structures can be synthesized? What kind of specific properties do superatomic molecules demonstrate? These are just some of our endless questions.
Won’t you join us in our adventure to elucidate these mysteries in nanochemistry?
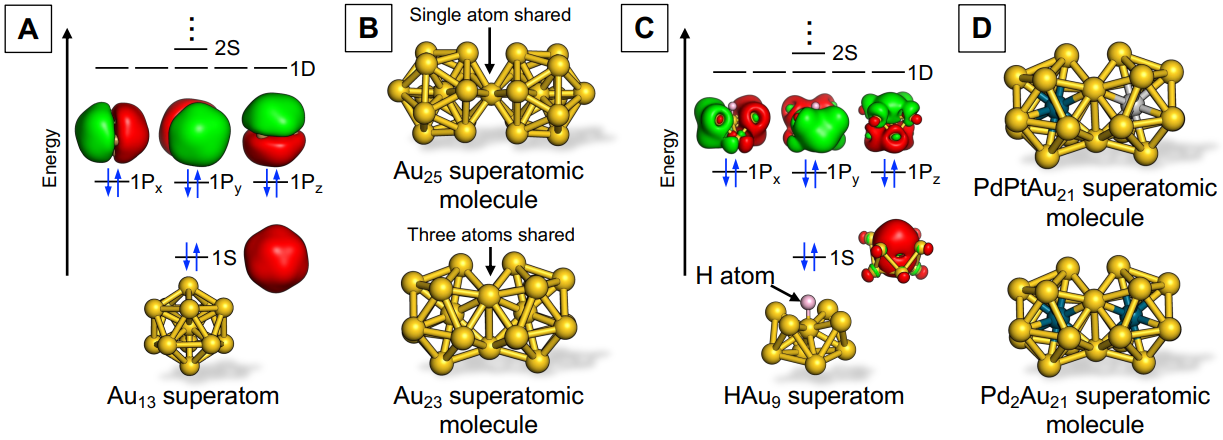
Figure: (A) Schematic diagram of a Au13 superatom(B)Example of a Au superatomic molecule(C)Schematic diagram of the structure of a HAu9 superatom, which is activated by combining Au9 with hydride (D)Example of an alloy superatomic molecule synthesized by a fusion reaction. Two electrons with opposite spins in each superatomic orbital are indicated in blue. The surfaces of the structures in the figure are entirely coated with organic ligands, which have been omitted for simplification. Figures courtesy of Assistant Professor Shinjiro Takano.
References
1) S. Takano, T. Tsukuda, “Chemically Modified Gold/Silver Superatoms as Artificial Elements at Nanoscale: Design Principles and Synthesis Challenges,” J. Am. Chem. Soc. 2021, 143, 1683.
2) E. Ito, S. Takano, T. Nakamura, T. Tsukuda, “Controlled Dimerization and Bonding Scheme of Icosahedral M@Au12 (M = Pd, Pt) Superatoms,” Angew. Chem., Int. Ed. 2021, 60, 645.
― This article is from the "Mysteries in Science" series in The Rigakubu News ―


