Exhibits
4D printed models of signaling membrane proteins
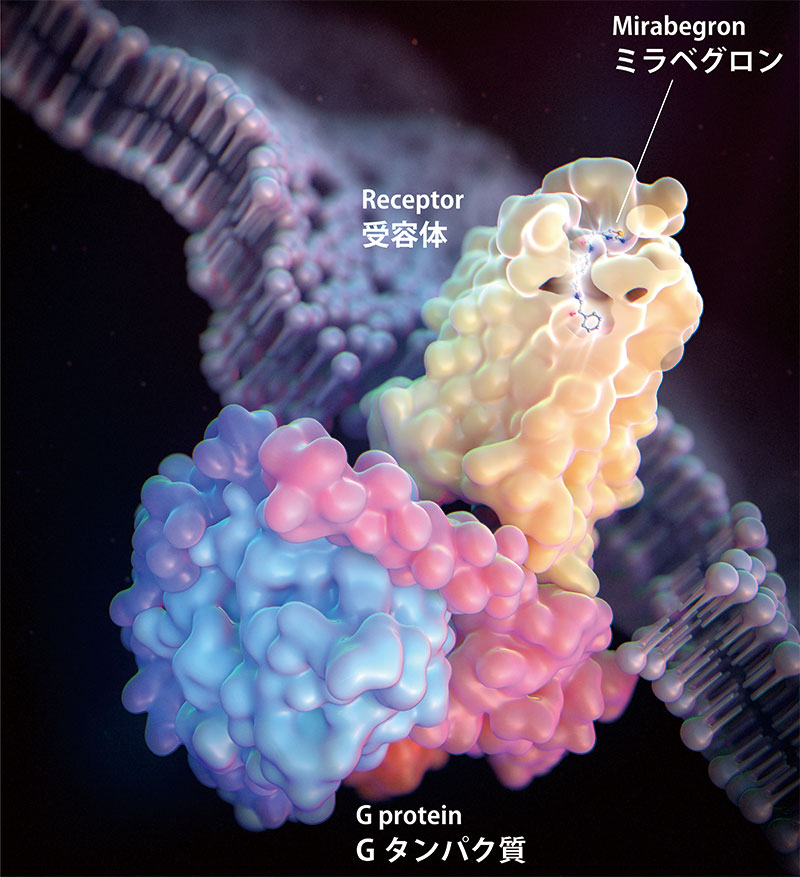
Figure 1: Structure of the mirabegron-activated β3 receptor-G protein complex
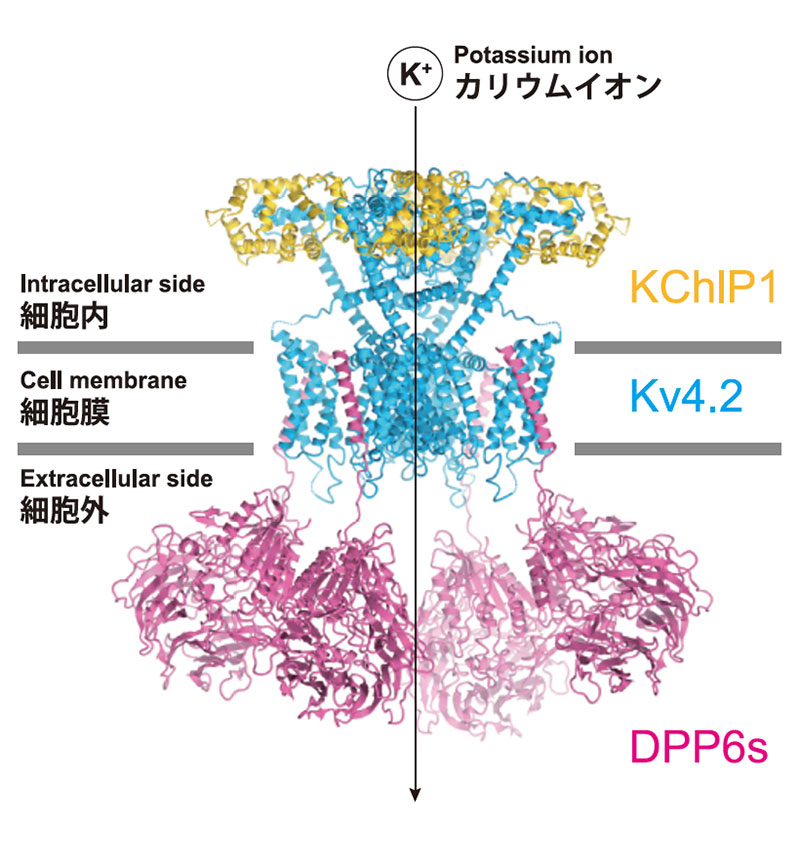
Figure 2: Structure of the Kv4-DPP-KChIP supramolecular complex
Multicellular organisms, including humans, must exchange information across cell membranes, which is controlled by membrane proteins. The Nureki Laboratory in the Department of Biophysics and Biochemistry, School of Science, The University of Tokyo, aims to elucidate the signal transduction mechanism at the atomic level by determining the three-dimensional structure of membrane proteins using cryo-electron microscopy.
The β3-adrenergic receptor is a G-protein-coupled receptor that receives ligands from outside the cell and activates G-proteins. β3-receptors stimulate sympathetic nerves and are responsible for fat burning and bladder relaxation. The Nureki Laboratory determined the structure of β3 receptors bound by the overactive bladder drug mirabegron and elucidated the mechanism of receptor activation (Fig. 1). This information will help design new β3 agonists with improved efficacy.
Voltage-gated potassium channel Kv4 is involved in the regulation of neuronal firing patterns, memory, and learning. The structure of the Kv4-DPP-KChIP supramolecular complex reveals a regulatory mechanism of gate opening and closing by the cofactors DPP and KChIP (Fig. 2, Kise et al., Nature, 2021), which can contribute to drug design for neurological diseases involving Kv4.
Responsibility for wording of the article
Osamu Nureki, Professor, Biophysics and Biochemistry [2022]