What rugby teaches about the researcher’s mindset
Hitosugi says that his dream, his only goal, is to discover room-temperature superconductors. Researching all-solid-state batteries, building a digital laboratory, and playing rugby – these are just ways to reach the ultimate goal.
Wait, did you say rugby?
“When I was a child, I lived in the US and grew fond of American football. I wanted to play a sport that used an oval ball, so I joined the rugby team at the University of Tokyo. I played as one of the backs. It was great fun. I injured my shoulder in my sophomore year and could not go all out anymore. However, even that is a good memory now.
Hitosugi says, and the next moment he smiles.
“I'm the current vice president of UTokyo's rugby team. In fact, I am trying to connect rugby with science research.”
But how could rugby be connected to room-temperature superconductivity?
“The decline in Japan's research prowess has become a major problem. Meanwhile, in the world of sports, including rugby and soccer, Japan can now compete with the world on an equal footing. If we can learn from sports how to nurture capable players and reform how science is conducted by integrating those principles, then Japanese researchers may be able to compete with the world's best, just as the Japanese national rugby and soccer teams are competing in the World Cup. For example, we have a lot to learn from how sports utilize digital technology.”
Hitosugi often talks about the combination of different areas. He says that combining different areas gives birth to something original and that it is vital to keep taking on challenges. The economist Joseph Schumpeter defined “innovation” as “new combinations.” Is Hitosugi trying to innovate by combining rugby with the mindset of young researchers?
“You can learn a lot from rugby. In rugby, you always have to get up and go forward even if you get knocked down. The rugby ball is oval, so when you kick it forward, it often comes back when it bounces off the ground. So, I hope that students will stand up for themselves and move forward again even if their experiments do not work out. I, myself, have failed many times. Even now, as I take on new challenges, I still often fail. However, learning from our mistakes allows us to take a step forward. Teamwork, a “one for all, all for one” mentality, is crucial in rugby. This mentality is also the same with collaboration in scientific research. The principle of “no side” is the most important. Even though we compete with each other body against body, we hold each other in high esteem. Once the game is over, we are instantly back to being friends. I think we need to have this attitude in science as well. I always try to be upfront when I think a certain line of discussion is incorrect. Voicing your opinion while respecting that of others, mutual acceptance is key. Going along with the majority or taking issue only in private is not the appropriate way to pursue science. The “no side” principle is important for science, too.”
Hitosugi says that he proactively tries to have such conversations with his students.
“Great rugby players often say that they used to practice judo or play soccer. It is their experiences in different areas that make them excellent rugby players. Accordingly, it is not enough to do research exclusively on superconductivity to discover superconductors. It is necessary to integrate ways of thinking from other disciplines. In Japan, they often say that the “nail that sticks out gets hammered down.” But I cannot endorse this notion. We need to respect each other’s originality (=the nail that sticks out). The shortcut to originality is “combining different areas.” If you start challenging yourself with new research topics in different disciplines, you might get confused at first. You might even regret doing it. You might feel that things are too difficult to understand and lose confidence in yourself. You might think that sticking to what you already know would be easier. But if you keep challenging yourself, gradually, your experiences will turn into strengths. The history of your challenges will be unique to you, and only to you. There are no two people who have walked the same path. Challenging yourself leads to your originality. I also have taken on many such challenges.”

Contributions to creating all-solid-state batteries
Hitosugi says do not be afraid to challenge yourself with different fields and areas.
“After graduate school, I worked for Sony as a researcher. But after a while, I started feeling I wanted to gain experience in other areas. So, I applied for a job in the marketing and sales department. I wanted to see how we can spread technologies and products to consumers. Every aspect of the work was new to me. Of course, I felt lost. However, I capitalize on those experiences even today. The major thing I learned is that any job, even product planning and sales, employ the same principles research does. It was a great discovery that made me realize that the wall separating different disciplines is not that high after all. I learned that because the principles are the same, I do not have to be afraid to challenge myself. I also learned to enjoy meeting new people and news ways of thinking while moving forward step by step.”
Hitosugi says combining different disciplines is the key to success in all-solid-state battery research. A battery’s structure consists of an electrolyte “sandwiched” between a cathode and an anode.
The electrolytes in lithium-ion batteries for smartphones and other everyday devices are liquid. However, the electrolyte in the next-generation batteries is solid. The technology not only reduces the charging time but also increases battery life. This is expected to revolutionize the world of batteries. Thus, the competition for the development of all-solid-state batteries around the world is fierce.
“Interface research is one of my areas of expertise. An interface is a surface where two materials contact each other. It is thanks to interface technologies that we can use smartphones to calculate or send emails. The interface is key within the battery itself as well. For example, unwanted substances formed at the electrolyte and cathode interface cause electrical resistance. In other words, ions are hindered in getting through, which was a problem in solid-state battery research and concealed the technology's true potential. However, we successfully created an interface that we perfectly controlled and investigated how low we could reduce the electrical resistance around 2013.”
Hitosugi used a cutting-edge technology he was already deeply familiar with: atomic-level thin-film synthesis. Using this technology, the lab produced an interface where the atoms were arranged in an orderly fashion. As a result, the lab successfully reduced the electrical resistance of the interface to one-fortieth of what was usual at the time, which was even less than half the interface resistance of liquid electrolyte lithium batteries. They demonstrated that the ions were moving fast through the interface, proving the viability of all-solid-state batteries.
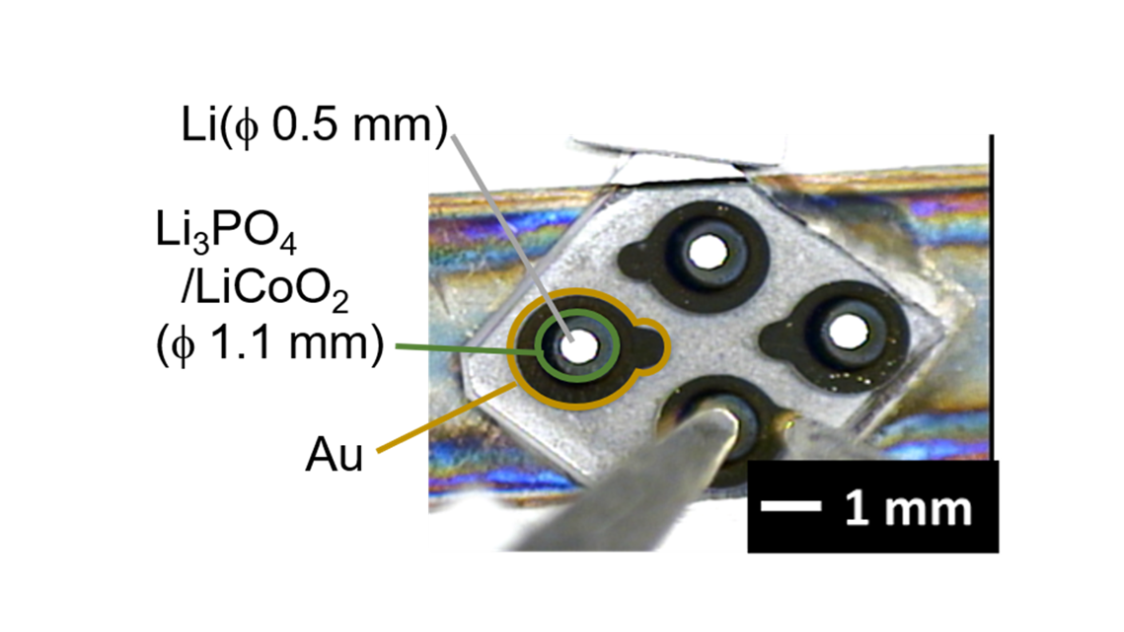
“When I started researching all-solid-state batteries, knowledge about solid-state physics and semiconductors had not yet been incorporated because they were thought of as different fields. So, we combined the knowledge of these fields, bringing interface-controlling technology from semiconductor research into electrochemistry. When we did that, ions started moving at surprising speeds. We successfully produced the most efficient battery in the world. It was proof that all-solid-state batteries have tremendous potential.”
When you read this, you may think that the research progressed very smoothly. Of course, that was not the case. Just like a bouncing rugby ball, it was a cycle of advances and setbacks. However, enjoying these cycles led to additional discoveries and research results in the form of the “digital laboratory.”
The birth of an AI-robot scientists
“The experimental work in research on battery interfaces is very labor-intensive. The tasks of materials science are delicate, and all work must be done by hand, one by one. It requires patience and time. I was wondering if there was a way to reduce the burden on researchers and accelerate research at the same time. Students still used the same techniques and procedures I used as a student, which I saw as a problem. I wanted young researchers to discover new materials I could not even dream about. Nonetheless, research was conducted the same way it was when I was a student. As an educator, I felt that creating copies of myself was not the right path. At that time, I came across another “different field,” mathematical sciences. As I had discussions with mathematical scientists, the idea of using AI and robotics to develop materials emerged, which I believed could innovate research. That was around 2014.”
The innovative idea was to create a small laboratory where an AI-controlled robotic arm could conduct experiments. It would be one integrated system that could autonomously repeat experiments and search for new materials aligned with the goals set by researchers. In other words, it is a digital laboratory with a robot scientist.
“At first, no one took me seriously, and I did not receive any research funding. However, we kept working on it bit by bit and managed to complete it thanks to the help of Associate Professor Ryota Shimizu and Project Assistant Professor Shigeru Kobayashi. In this digital laboratory, when a researcher sets a goal, the AI creates the experimental design, and the robot conducts repeated cycles of experiments and evaluation. The results are fed back to the AI, which then plans the next round of experiments for the robot to execute. In my lab, we are collaborating with AI and robots to pursue finding new materials.”
Hitosugi is a global pioneer in the digitalization of materials science, a branch now called materials informatics. Even today, the small, silvery, UFO-like robotic scientist is working diligently in Hitosugi’s lab.
“Japan is a leader in solid-state materials and materials science, both in research and industry. We are working hard with various companies to accelerate research because the world is catching up fast.”
However, as the opening suggested, this digital laboratory, too, is just a way to get closer to the ultimate goal of discovering room-temperature superconductors.
“The way we do science has been changing rapidly, and now, like in the digital laboratory, we have robots doing various experiments for us. The new methods open a much wider range of possibilities to explore new materials. There is a vast search space for materials science, and digital laboratory raises the probability of finding superconductors. This makes me incredibly excited. It is similar to the feeling I had when I got my driver's license for the first time. I felt like I could get in a car and go anywhere in the country and that the range of possible activities had expanded dramatically. It made me incredibly excited. Using AI and robots, we can go to many places in the vast search space of materials science!”
Hitosugi says that in that vast search space, there must be as many unknown materials useful to society as stars in the sky, giving birth to many dreams in materials science.
The inevitable spread of solid-state technology
The high-temperature superconductivity “fever” around the time he was a high school student inspired Hitosugi to pursue a career in science. The “fever” began in the mid-1980s with the appearance of cuprate superconductors that reached superconductivity at relatively high temperatures (although still below zero degrees Celsius), followed by a surge of interest in superconductivity worldwide. After entering the Faculty of Engineering at the University of Tokyo, Hitosugi joined the laboratory of Koichi Kitazawa (Professor Emeritus, The University of Tokyo, 1943-2014), who was said to be the originator of the high-temperature superconductivity “fever.” Hitosugi's dream began there.
“Discovering room-temperature superconductors could solve all sorts of problems - environmental problems, energy problems, transportation problems, and so on - all at once. It is quite a dream, isn't it? I've always wanted to pursue such a dream.”
However, the road to realizing this dream is still unclear. Not just to Hitosugi but to every scientist in the world: nobody knows where the goal line is.
“A material was recently discovered that could reach superconductivity at temperatures close to zero degrees Celsius, although it requires the application of very high pressure. Many superconductors have been found that BCS theory, a widely used theory of superconductivity, cannot explain. It would be interesting if a not-yet-known mechanism could bring about superconductivity. So, I am aiming to find unexpected results. It would be boring if experimental results were as expected, wouldn’t it? In the end, I want to aim for the unexpected. Discovering superconductors from research on solid-state batteries would be unexpected indeed. I want to approach superconductivity from a perspective nobody else does.”
Hitosugi writes the following on his laboratory’s website.
“Solids are interesting. When atoms and molecules form a solid, physical properties that were previously unimaginable emerge.”
Hitosugi sees infinite possibility in solids, materials created by “metamorphosis.” That is why solids are interesting.
He picks up a lump of silicon sitting on his desk and says.
"This silicon lump originates from rocks lying around in the vicinity. Amazingly, pieces of these solids can do machine learning and calculations. This is the interesting thing about solids: they are the building blocks of many devices. There are only two devices in this laptop that are not solid. One is the lithium-ion battery, and the other is the LCD. Many other devices have had such “solid” innovations.
For example, candles were replaced by gas lighting (using gas), which was then replaced by electric lightbulbs (using vacuum). Then, they were replaced by white LEDs (using solids). The same is true for displays: LCDs replaced cathode-ray tubes but were later replaced by OLEDs made of solids. The trend toward solid devices is very straightforward. The reason is that solid-state devices are more energy-efficient and safer because they are hard to damage. Therefore, the shift to solid-state batteries is quite appropriate from a technological perspective.”
When asked for a message to the younger generation, Hitosugi says the following.
“It is important to take on challenges. I would like young people to challenge themselves more without being confined to isolated worlds. Individuality is not something you are born with; it is something you build. When you take on challenges and make mistakes, you learn many things and become capable. You assemble your personal toolkit, so to speak. When you combine your experiences, you can create something that only you can do, in other words, something original. I hope young students and researchers keep challenging and widen their horizons.”

Hitosugi, too, continues to challenge himself. With the robot scientist by his side, he ventures into the vast search space for the ultimate material - the room-temperature superconductor.
※Year of interview:2023
Interview/Text: OTA Minoru
Photography: KAIZUKA Junichi

![リガクル[RIGAKU-RU] Exploring Science](/ja/rigakuru/images/top/title_RIGAKURU.png)