In His Twenties, Totally Absorbed in the Synthesis of Toxins
Our world is made up of 118 types of atoms, many of which are bound to each other in different ways to form a wide variety of molecules. More than one billion molecules are known to exist, but there are as many types of molecules as stars in the night sky, and that number is growing every day, thanks to chemists like Professor Hiroki Oguri.
“There may be molecules that could change the world that don’t yet exist on Earth or even elsewhere in the universe. We can envision such molecules and create them with our own hands. That’s the attraction of chemistry and its overwhelming advantage.” In fact, that’s the challenge Oguri is taking up in his laboratory on the University of Tokyo’s Hongo campus. “We can create a limitless number of new molecules. Soon there may be a new gold rush of world-changing molecules that will benefit humankind.”
Growing up in Tokorozawa in Saitama Prefecture, Oguri loved collecting insects in the wooded area that’s now known as Totoro’s Forest and fishing in the neighborhood pond. He recalls, “I joined the Department of Chemistry in the Faculty of Science at Tohoku University from Kawagoe High School, and before I knew it, I found myself involved with fish again in graduate school.” He worked on the chemical synthesis of ciguatoxin, a poison that accumulates in tropical fish and shellfish. Ciguatoxin is responsible for the foodborne illness known as ciguatera fish poisoning, and is 100 times more toxic than tetrodotoxin found in pufferfish (known in Japan as the delicacy fugu).
“My mentor at Tohoku University, Professor Masahiro Hirama, gave me the opportunity to tackle chemical synthesis while determining the molecular structure of ciguatoxin. I found it really tough at the beginning, but the more I got into it, the more I enjoyed it and I eventually became totally absorbed in the work. In my master’s program, I hadn’t envisioned a future as an academic researcher, but ultimately, I gambled my entire twenties on it.”
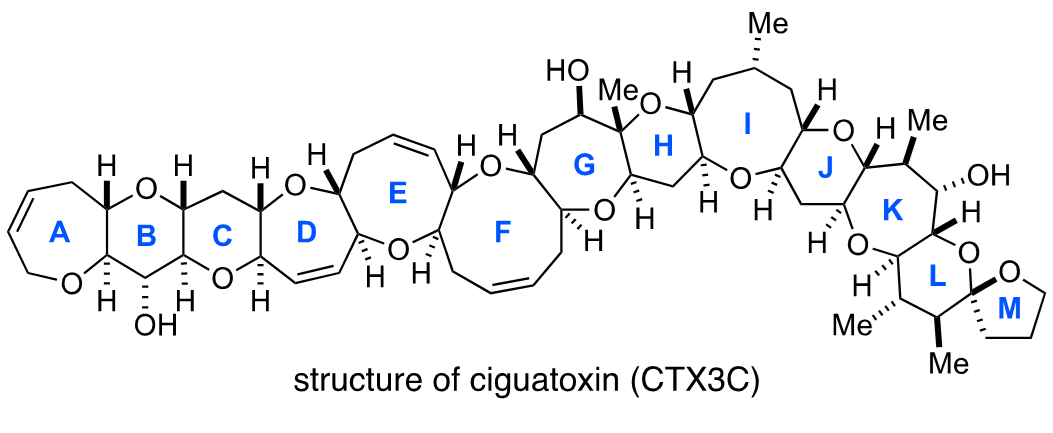
Oguri and his colleagues embraced the challenge of achieving the total synthesis of ciguatoxin. Total synthesis is the artificial chemical synthesis of natural products from the simplest starting materials. In other words, chemists attempt to produce organic molecules in flasks and other laboratory apparatus without the help of nature. Since ciguatoxin was an extremely complex and huge molecule with a ladder-shaped structure composed of 13 ether rings, there were high barriers to its synthesis. Of course, if the total synthesis of ciguatoxin could be achieved, it would be a world first. So how did it turn out?
“In the Hirama Laboratory, our team continued to take on challenges for ten years with more than ten colleagues, including Toru Oishi1 and Masayuki Inoue2, eventually achieving our goal of total synthesis. Based on our synthetic method, we prepared a monoclonal antibody that recognizes ciguatoxin (CTX3C) and then developed a method for detecting the toxin. Although we were in our prime years for enjoying ourselves, Mitsuru Shoji3 and our other junior members devoted themselves to the chemical synthesis of the huge toxin through repeated trial and error, from morning to night. I genuinely enjoyed our day-to-day experiments for assembling complex molecules by the synthetic routes we’d devised. That’s why I would like to tell students that they should do whatever they find enjoyable, regardless of what others say. Go all out through your twenties, pursuing your own interests!”
This experience of ‘going all out’ became Oguri’s starting point.
Creating Molecular Skeletons
After starting his career as a researcher, Oguri eventually left Japan for Harvard University in Cambridge, Massachusetts, USA. “Professor Stuart L. Schreiber, a chemist who specialized in the chemical synthesis of natural products, had begun the challenge of creating organic compounds that could control the function of every single protein in the human body. I resonated with Professor Schreiber’s perspective that synthetic chemistry to generate molecular diversity is the key to opening the door to the future.”
Professor Schreiber is also known for pioneering the new field of chemical biology, which has been predicted may earn him a Nobel Prize.
Most organic compounds have a molecular skeleton that is formed primarily by carbon atoms bound together. We can think of the “molecular skeleton (or simply skeleton)” as corresponding to the spine in a human, or the chassis and engine in an automobile. Other atoms such as oxygen, nitrogen, and sulfur can be appended to this skeleton in different ways, creating a huge variety of organic compounds with diverse properties. In the development of drugs, agrochemicals, and fine chemicals, it is generally difficult and important to obtain an appropriate molecular skeleton, and the subsequent chemical synthesis is relatively straightforward.
“At Harvard, I independently devised and developed a concise synthesis process to generate complex molecular skeletons like those of natural products in just a few steps, through the assembly of four simple starting materials. Through this research, I was able to publish a paper co-authored by myself and Professor Schreiber, with whom I’d longed to work,” said Oguri with a satisfied smile.
Synthetic Chemistry that Mimics and Leverages Biosynthesis
Despite Professor Schreiber’s pleas for him to stay, Oguri returned to Japan after only one year at Harvard because of his desire to pursue an “Oriental approach” different from that of Western scientists, or in Oguri’s words, “a synthetic approach that is more akin to Japanese natural product chemistry, or alternatively, one that is in harmony with nature.”
“Since Western researchers are good at rational approaches, I presumed that they would develop synthetic processes to efficiently generate many kinds of molecules by adopting efficient chemical reactions suitable for expanding structural diversity. In contrast, I wondered whether it might be possible to mimic the biosynthetic processes of plants and fungi, which produce unique natural products, to create molecules that do not exist in nature through the power of synthetic chemistry. I also considered the potential for recruiting algae and fungi as ‘collaborators’ so that anyone can easily access molecular skeletons that could previously only be synthesized by only a handful of laboratories using chemistry alone! I had envisioned that such a pioneering and unique chemical approach, which is distinct from the one by Western researchers, would allow us to leverage the strengths of Japanese chemists of natural products”
For example, ciguatoxin is produced by a type of plankton known as dinoflagellates. When it comes to producing ciguatoxin, these dinoflagellate algae which live on coral reefs are, in Oguri’s words, “vastly greater ‘chemists’ than us.” Biosynthesis is the process by which living organisms use enzymes as catalysts to produce natural organic compounds in their own bodies, just as dinoflagellates produce ciguatoxin. Oguri was aware of both the importance of research to elucidate a series of chemical reactions known as biosynthetic pathways that occur in the bodies of these organisms and the untapped potential of approaches to artificially synthesizing useful natural products,
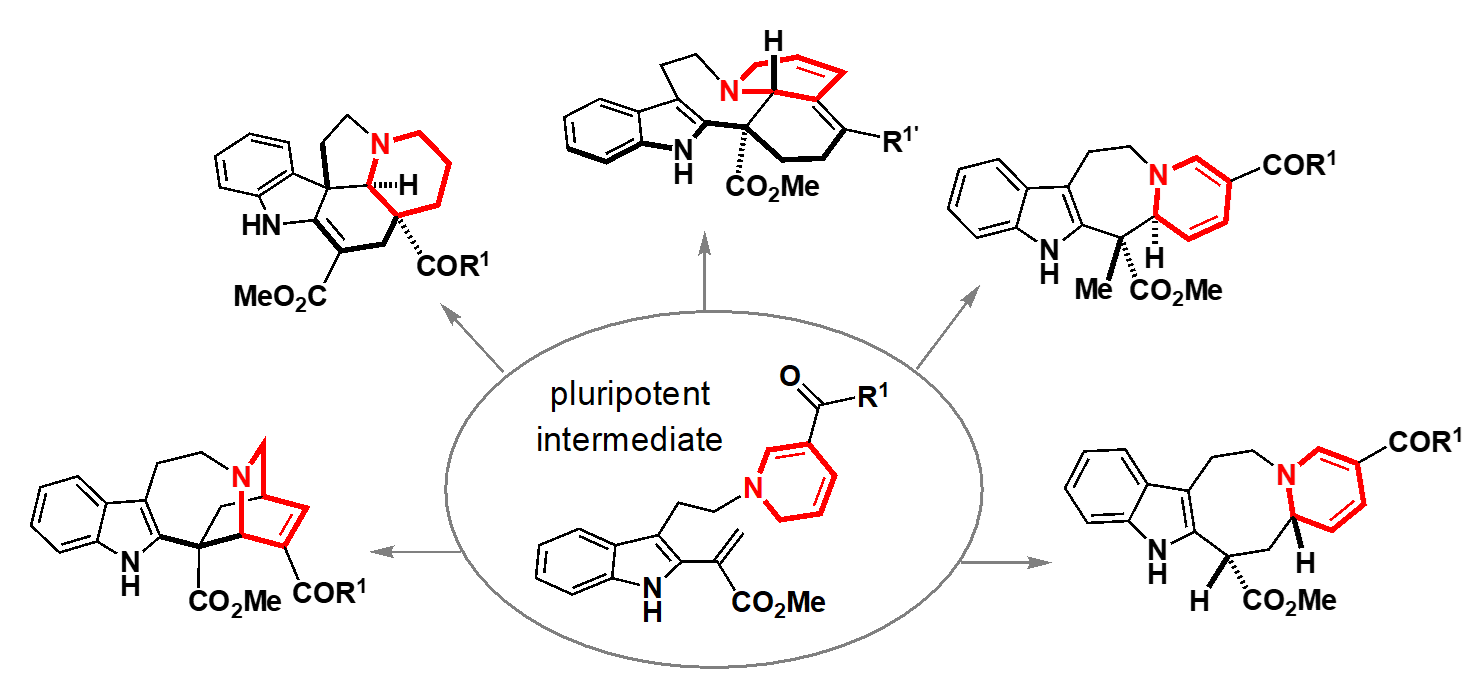
“This enthusiastic research outlook led to my hiring as an assistant professor by Professor Hideaki Oikawa of the Department of Chemistry in the Faculty of Science at Hokkaido University, and so began my research life in Sapporo. In the Oikawa Laboratory, I was able to learn about the biosynthesis of diverse natural products, from the fundamentals to the cutting edge of research.” At Hokkaido University, Oguri successfully developed an approach that focused on the alkaloids (natural products containing nitrogen atoms) produced by Vinca plants. Plants such as the Vincas employ a biosynthetic strategy in which a common key intermediate (a compound in a synthetic pathway) with versatile chemical reactivity is generated, and different enzymes act on this intermediate to produce diverse molecular skeletons. However, this key intermediate is so unstable that attempts to extract it from plants or to chemically synthesize it have not been successful. Oguri and Haruki Mizoguchi4 designed and rapidly synthesized a ‘pluripotent intermediate’ that mimics this key biosynthetic intermediate, is stable during the handling in the laboratory, and can be differentiated into various molecular skeletons. By reacting this pluripotent intermediate generated in vitro under various conditions, they succeeded in generating a set of five different molecular skeletons. By mimicking the biosynthetic process of the Vinca plants, they were then able to conduct both rational and playful experiments in their flasks, obtaining a certain response that this synthetic approach allows the researcher to concisely and flexibly generate drug candidate compounds that were previously inaccessible.
Pioneering Chemo-Enzymatic Hybrid Synthesis
As an independent professor at Tokyo University of Agriculture and Technology, Oguri pioneered a synthetic method that utilizes biosynthetic enzymes to assemble complex molecular skeletons in a single step. This was the development of a chemo-enzymatic hybrid process for saframycins.
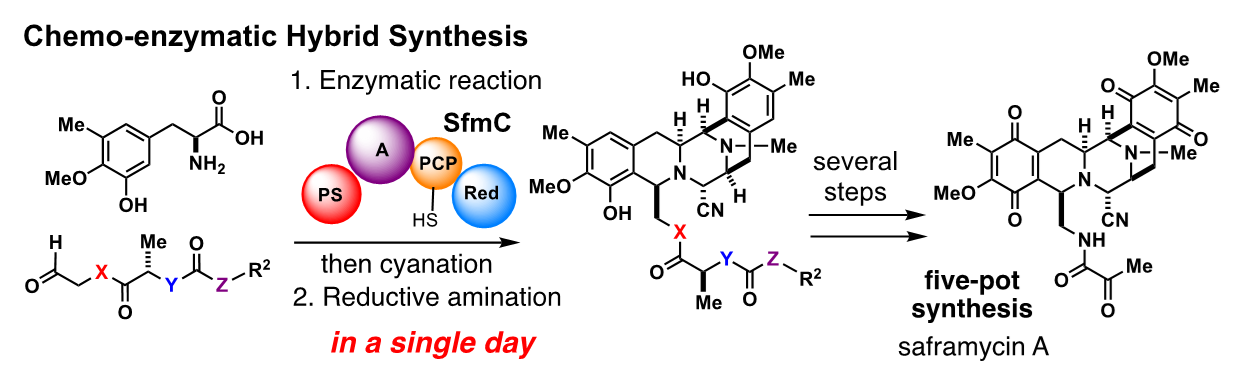
Saframycin and ecteinascidin are both natural products that exhibit potent anti-cancer activity and share a common molecular skeleton. Ecteinascidins isolated from the marine organisms known as ascidians (sea squirts) have been clinically used for the treatment of intractable cancer, but more than 20 steps are required for their chemical synthesis, and the generation of new derivatives is challenging. Leveraging the research on saframycin biosynthesis conducted in Oikawa’s laboratory at Hokkaido University, Oguri and Ryo Tanifuji5, who was a graduate student at the time, took on the challenge to assemble the common molecular skeleton in one pot by conversions of chemically synthesized substrates with a biosynthetic enzyme expressed in the bacterium Escherichia coli (E. coli).
“This biosynthetic enzyme is a huge protein, with a molecular weight – the sum of the atomic weights of the atoms that make up the molecule – of 160,000. This organism purposely produced huge enzymes to biosynthesize saframycin, which has a molecular weight of only about 500. Presumably, to securely construct a small but crucial skeleton for the survival of the species through an elaborate series of seven consecutive chemical reactions!”
Every day from early in the morning, Tanifuji purified the massive enzyme expressed in E. coli and conducted the enzymatic conversions of the synthetic substrates with great persistence. The days were dark, with no progress in enzymatic conversions, but a breakthrough was made thanks to keen observations by Tanifuji, who had by now begun his doctoral program. After making improvements and solving numerous problems, he was finally able to synthesize a complex molecular skeleton just in a single day.
“From there, through two to three steps of chemical conversions, we also succeeded in the total synthesis of the natural products jorunnamycin and saframycin. Through the development of an artificial biosynthetic process, chemo-enzymatic hybrid synthesis allows the very rapid assembly of the considerably complex skeleton, which is important as a seed molecule for pharmaceuticals. Thanks to this breakthrough, “Tanifuji has become a renowned young chemist,” says Oguri with a smile.
Creating Yet-Unexplored Molecules with Far Fewer Synthetic Steps
Concurrently with investigating the hybrid synthesis of saframycins, Oguri also has been exploring other projects in his laboratory. One of these is the development of a rapid synthetic process by modification of the molecular skeleton of a natural product known as artemisinin, an active ingredient in Chinese herbal medicines that has revolutionized the treatment of malaria. Dr. Tu Youyou, a Chinese female researcher, won the 2015 Nobel Prize in Physiology or Medicine for developing a malaria treatment using artemisinin. Artemisinin is biosynthesized by Artemisia (wormwood) plants. Currently, artemisinin-based drugs are manufactured through artificial large scale fermentation of a biosynthetic intermediate employing an engineered yeast with incorporation of the biosynthetic gene from the Artemisia plant and subsequent six-step chemical manipulations. Oguri sought to make artemisinin from a completely different approach.
“We designed a molecule called 6-aza-artemisinin, in which one of the carbon atoms in the artemisinin skeleton was replaced by a nitrogen atom. Incorporating a nitrogen atom into the backbone enabled us to develop a new synthetic route that is distinct from existing production processes which had to rely on enzymatic conversions. The molecular skeleton responsible for the remarkable therapeutic effects can be generated from simple starting materials in just four steps.”
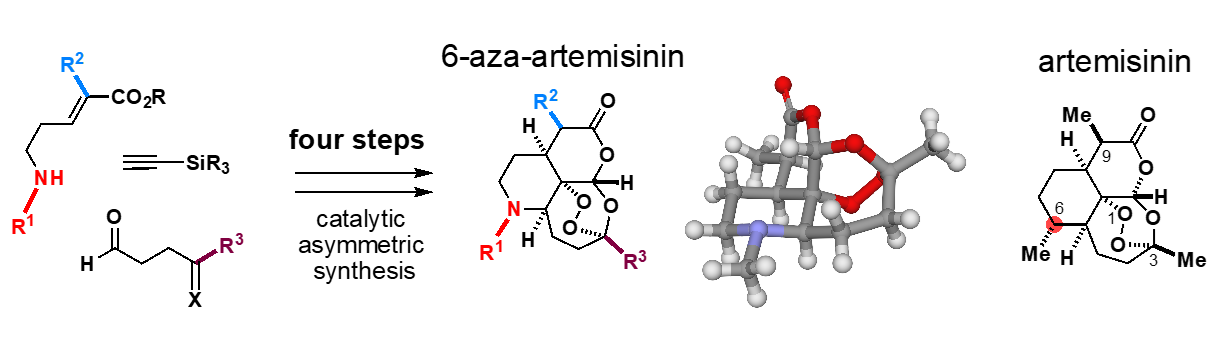
“Although approaches for generating synthetic analogs of complex natural products by simplifying their structures have been studied, creating more functionalized molecular skeletons by replacing elements in the molecular skeleton without simplifying the structure of the natural product have been largely unexplored. In fact, we were able to demonstrate that aza-artemisinin is more effective in treating malaria than existing artemisinin-based drugs. By envisioning a new molecular framework and creating it with the power of chemistry, we can provide a springboard for new developments in medicine and pharmaceutical science.”
Pursuing Innovations from Basic Research
Oguri and his colleagues aren’t simply setting out to synthesize new molecules. They are also seeking to create unique value through the ‘chemical evolution’ of natural products exploiting cutting-edge chemistry to generate new molecular skeletons that no-one has ever seen before. Creating unexplored molecular resources will lead directly to the generation of candidate compounds for next-generation drugs and agrochemicals.
“As seen with artemisinin, synthesizing a molecule with a complex structure and developing it into a drug are a challenging task. From the business perspective, the decision to proceed with the natural-product based drug candidates tends to meet resistance unless the drug can be reliably synthesized or easily cultured. As a result, pharmaceutical companies have shied away from natural product-based drug discovery research over the past 30 years. However, if we can easily construct molecular skeletons that promise superior efficacy, and achieve flexible chemical modifications for optimizing the drug candidates, the rationale and unexplored potential of natural-product based drug discovery, which is imbued with a history of biological evolution, will demand a fresh look.”
Oguri says his ambition as a university researcher is to pursue basic research that leads to new ways of thinking and different strategies for designing and synthesizing biologically intriguing molecules. While learning from nature and utilizing biosynthetic intermediates and enzymatic reactions, he seeks to develop versatile synthetic processes that can be leveraged at will. As a chemist, Oguri’s key focus is to show that anyone can easily create complex molecular skeletons by using these approaches, and that there are many possibilities and developments that lay beyond. This may be a more socially conscious and sincere endeavor that goes beyond the mere discovery of drug candidate compounds.
Oguri’s laboratory boldly creates molecules that do not exist on earth, and at an astonishing pace. “In the drug discovery field, many kinds of molecules have been synthesized by combining building blocks like flat panels. However, conventional methods are limited by the complexity and diversity of molecular structures. In plants and fungi by contrast, massive enzymes are made from biosynthetic genes encoded as extremely long genome sequences. Elaborate multi-step enzymatic conversions thereby enable biosynthesis of natural products with complex, intricate molecular surfaces. The chemical synthesis of natural products and their analogs that interact with inherently bumpy molecular surfaces of drug target proteins with high affinity, is much more challenging, time-consuming, and labor-intensive, and therefore has been difficult to achieve. Our laboratory continues to address the synthetic challenges to creating the complexity and diversity of these biologically relevant molecules in a systematic and programmable manner. If we can make it easier for anyone to synthesize complex molecules, this could be a game changer in the world of drug discovery.”

Aiming for Unexpected Discoveries
One of these ‘unexplored research areas’ involves the rational generation of complexity and diversity for molecules that promise to exhibit useful biological activity, and the manipulation of the three-dimensional structure of molecules at will. Synthesize the molecules you have in mind at your own command. Oguri notes that this will contribute not only to drug discovery, but also to the elucidation of the mysteries and mechanisms of living organisms. This is the area of chemical biology to which Oguri is most committed. When asked what new discoveries he would like to make in that research area, he replied.

“I want to create unique molecules that no one has even imagined before, molecules that elicit the response ‘How can a small molecule induce such a big change!?’ It may sound a little silly, but if it’s not like that, it won’t have an impact, and it won’t be a ‘real discovery.’” Oguri laughed mischievously.
And the last question: do you enjoy research?
“It’s a lot of fun. We are routinely making misjudgments and frustrating mistakes throughout our daily experiments, but once you experience the real thrill of research, you can never stop exploration! Did everything go as planned? Why did such a phenomenon occur? By taking in the message that the flask in front of our eyes tells us, we can improve and make steady progress. At our Hongo laboratory, we hope that students will send their own hand-created precious molecules to living organisms, listen to the whispers between cell, and make unexpected discoveries!”
1: Currently Professor at Kyushu University; 2: Currently Professor at the University of Tokyo; 3: Currently Professor at Yokohama College of Pharmacy and Pharmaceutical Sciences; 4: Currently Associate Professor at Okayama University; 5: Currently Assistant Professor in the Oguri Laboratory.
※Year of interview:2022
Interview/Text: Minoru Ota
Photography: Junichi Kaizuka

![リガクル[RIGAKU-RU] Exploring Science](/ja/rigakuru/images/top/title_RIGAKURU.png)










