There are a beaker and a flask that have been handed down for generations in the Solid State Physical Chemistry Laboratory of the Department of Chemistry (Prof. Shin-ichi Ohkoshi). A pale purple liquid that reminds us of Japanese wisteria and a liquid as bright red as a ruby ... Both are “colloidal gold solutions” with gold nanoparticles floating inside.
A colloid is a substance made up of a system of nanoscale particles dispersed in a medium. If the medium is liquid, it is called a colloidal solution. Good examples around us would be milk and Indian ink. Floating in milk are particles of milk fat while soot carbon particles drift in Indian ink.
On the labels that make us feel the passage of time are written “Gold Sol … Prepared by von Weimarn (1923) (photo left below) and “Prepared by Prof. Sameshima”. “May 23, 1933. Gold Sol” (photo right below).
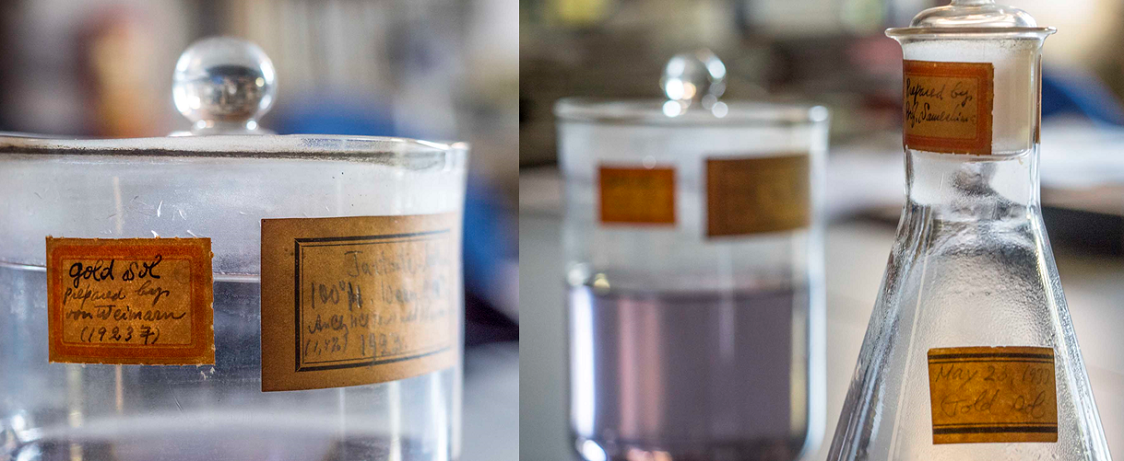
We can see that the former was made by Weimarn in 1923 and the latter by Prof. Sameshima on May 23, 1933. Other records show that the former was prepared before the Great Kanto Earthquake of Sept. 1. Weimarn’s colloidal solution survived the devastating earthquake sitting in the first reinforced concrete building of the University of Tokyo that housed the Department of Chemistry at the time.
Weimarn is the name of a great authority on colloid science born in Russia. He fled to Japan from the Soviet Union in 1921 after the Russian Revolution. The man he sought after in Japan was Prof. Kikunae Ikeda who was teaching a physical chemistry class in the Department of Chemistry. Prof. Ikeda is well known for his discovery of monosodium glutamate that produces umami. He studied in Britain under Friedrich Wilhelm Ostwald, one of the modern founders of physical chemistry, and laid the foundation for the study of physical chemistry in Japan. Ostwald’s son was also a renowned scholar in colloidal research and a close friend of Weimarn’s. That relationship prompted Weimarn to travel to Japan.
In 1923, Prof. Jitsusaburo Sameshima, otherwise known as “Prof. Samesima,” took up his position in the Department of Chemistry and taught “Chemistry Course No. 1” renamed from “Physical Chemistry Course.” It is believed that Weimarn worked on the colloidal solution under Prof. Sameshima at the time. Prof. Sameshima became the foremost authority in colloid science in Japan, leaving behind his major work, the Japanese translation of Colloidal Science.
It is well known that colloidal gold appears red when the size of its gold particles is around 10 nm in diameter and becomes increasingly blue as the size grows, forming a muddy yellow solution when it exceeds 100 nm. It is estimated that gold particles of about 20 to 90 nm in diameter are dispersed in the solution prepared by Weimarn. “Colloidal gold” is also known as “Gold Sol” and its English name appears on the label. Sol means a colloidal solution which has low viscosity and retains the characteristic of fluid.
Colloid science is one of the important themes in physical chemistry. Colloidal particles do not precipitate in a medium and continue to disperse and float. This movement is known as “Brownian motion” and is described by equations today. The colors of the solutions that have remained unchanged for 90 years proves the correctness of colloid science.
“Chemistry Course No. 1” taught by Prof. Sameshima was later renamed the “Solid State Physical Chemistry Laboratory.” Shin-ichi Ohkoshi, the sixth professor in charge of the laboratory, has further developed physical chemistry that Prof. Ikeda and Prof. Sameshima helped take root in Japan. The results in colloid chemistry are also used in the synthesis of new substances.
Interview and text: Masatsugu Kayahara
Photography: Junichi Kaizuka
Originally published in The School of Science Brochure 2018

![リガクル[RIGAKU-RU] Exploring Science](/ja/rigakuru/images/top/title_RIGAKURU.png)









