DATE2021.09.23 #Press Releases
Atomic-level structures illuminate how macromolecular ion channel complexes work in the brain
Overview of the press release
Using cryo-electron microscopy, scientists from the University of Tokyo’s Graduate School of Science and their colleagues have determined the structures of Kv4 macromolecular complexes at the atomic-level and revealed how regulatory subunits modulate Kv4 channel gating.
Many ion channels are large macromolecular protein complexes composed of pore-forming subunits and regulatory subunits which modulate and fine-tune channel properties for physiological function. Researchers in the ion channel field have been working on elucidating how regulatory subunits modulate the gating of ion channel complexes and have provided working models. However, without direct visualization of the structures of ion channel complexes at the atomic-level, the models remained hypotheses until now.
Voltage-gated potassium (Kv) channels in neurons play a crucial role in shaping and transducing electric signals in the brain. Kv4 channels are macromolecular complexes which contain two regulatory subunits: KChIPs and DPPs. These regulatory subunits fine-tune Kv4 properties by modulating the Kv4 channel gating kinetics.
“Sometimes, regulatory subunits with similar structural elements confer totally distinct properties to their common pore-forming subunit,” said Yoshiaki Kise, a project associate professor in the Department of Biophysics and Biochemistry at the University of Tokyo. “We were very interested in how such functional diversity of the ion channels is obtained by the regulatory subunits. To find the answer, we needed to visualize the ion channel complex at the atomic resolution.”
The molecules of biological protein complexes are too small to visualize with an optical microscope using visible light. Therefore, in this study, the research team used cryo-electron microscopy to determine the structures of Kv4 channel complexes at the atomic resolution. Cryo-electron microscopes use electron beams with wavelengths 100,000 times shorter than that of optic light, which allowed the research team to magnify and visualize the molecules of Kv4 channel complexes.
“The structures of Kv4 macromolecular complexes resolved in our study beautifully reveals not only the features hypothesized previously, but also unexpected features which thoroughly explain the modulatory mechanisms,” said Kise. “However, we just unraveled a part of the gating modulation of ion channel complexes because all the structures adopted open activated conformation. Our next challenge is to capture the resting and inactivated conformation of the ion channel complexes to draw a whole picture of the gating cycle modulated by regulatory subunits.”
The pore-forming subunits and the regulatory subunits are linked to various mental disorders including autism spectrum disorder and schizophrenia. Gaining an atomic-level understanding of the modulatory mechanisms of the ion channel gating by regulatory subunits could contribute to the development of novel drugs to treat these disorders.
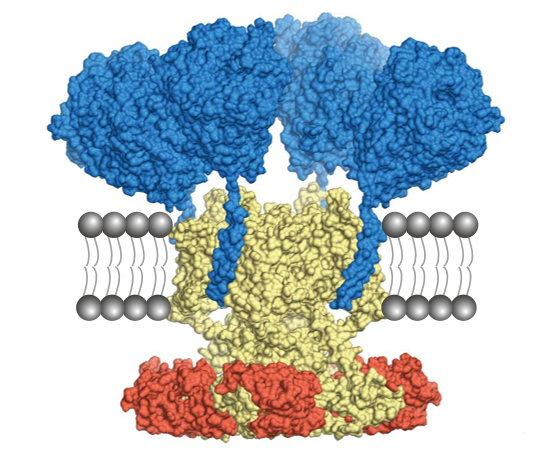
Figure: Cryo-EM structure of Kv4-KChIP-DPP macromolecular complex.
Kv4: yellow, KChIP: red, DPP: blue. The complex is hypothetically embedded in the lipid bilayer (gray).
Research Team
Yoshiaki Kise, Project Associate Professor at the Department of Biophysics and Biochemistry, The University of Tokyo.
Osamu Nureki, Professor at the Department of Biophysics and Biochemistry, The University of Tokyo.
Publication details
Journal Nature
Title Structural basis of gating modulation of Kv4 channel complexesAuthors Yoshiaki Kise*#, Go Kasuya*#, Hiroyuki Okamoto, Daichi Yamanouchi, Kan Kobayashi, Tsukasa Kusakizako, Tomohiro Nishizawa, Koichi Nakajo & Osamu Nureki# DOI 10.1038/s41586-021-03935-z
Paper link


